Six trials used an intention-to-treat analysis. The number of participant drop-outs was acceptable in the majority of the trials. Other biases that existed in the trials included: early stopping of AbMole Mepiroxol lenalidomide maintenance therapy based on an increased incidence of adverse events; early trial unblinding and crossover; trial designed and data analyzed by the manufacturer of lenalidomide; and patients receiving inappropriate doses of steroid treatment. During the last five years, a number of RCTs have examined the efficacy and safety of lenalidomide for the treatment of MM. Hence, we performed a meta-analysis in an attempt to gain further insight into the efficacy and safety of this treatment. A total of seven RCTs met the criteria for inclusion in our meta-analysis. The included trials were heterogenous in terms of inclusion criteria and treatment regimens; however, our overall analyses revealed that lenalidomide therapy significantly improved the rates of complete response and partial response and, importantly, increased progression-free survival relative to placebo/control. These findings were consistent among all RCTs included in our study. In contrast, lenalidomide significantly increased the risk of several adverse events, specifically neutropenia, deep vein thrombosis, infection, and hematologic cancer. More recent studies report conflicting results. Gay et al. retrospectively studied 411 patients to compare the efficacy and toxicity of lenalidomide plus dexamethasone versus thalidomide plus dexamethasone as initial therapy for newly diagnosed myeloma. In that study report, patients receiving lenalidomide plus dexamethasone had a longer time to progression, progressionfree survival, and overall survival than the group receiving thalidomide plus dexamethasone. A recent observational study assessed the efficacy and safety of lenalidomide plus dexamethasone in patients with relapsed or refractory MM who had been previously treated with thalidomide; the group receiving lenalidomide plus dexamethasone experienced a higher overall response rate, longer time to progression, and progression-free survival compared to those receiving placebo plus dexamethasone, despite prior thalidomide exposure. Clearly, further RCTs are needed to determine if specific lenalidomide treatment regimens 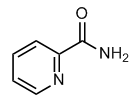 and/or patients characteristics are more likely to result in significantly increased overall survival. In addition to efficacy, safety is an equally important consideration for any chemotherapeutic agent. Obviously, the balance of any treatment must favor benefit over harm. The majority of adverse events reported in the studies we evaluated are manageable and do not appear to outweigh the benefits of treatment. Neutropenia and other hematologic toxicities can be managed with dose adjustment and/or treatment with granulocyte colony stimulating factor. Thromboprophylaxis is clearly indicated for patients being treated with lenalidomide to ameliorate the risk of deep vein thrombosis and other thrombolytic events.
and/or patients characteristics are more likely to result in significantly increased overall survival. In addition to efficacy, safety is an equally important consideration for any chemotherapeutic agent. Obviously, the balance of any treatment must favor benefit over harm. The majority of adverse events reported in the studies we evaluated are manageable and do not appear to outweigh the benefits of treatment. Neutropenia and other hematologic toxicities can be managed with dose adjustment and/or treatment with granulocyte colony stimulating factor. Thromboprophylaxis is clearly indicated for patients being treated with lenalidomide to ameliorate the risk of deep vein thrombosis and other thrombolytic events.