The minimal ability of the adult human heart to regenerate lost or damaged cardiomyocytes has led to an intense effort to direct human embryonic stem cells to form cardiomyocytes in order to model human heart disease and develop therapies. hESC-derived cardiomyocytes resemble immature human fetal cardiomyocytes by multiple criteria, including electrophysiology, calcium handling, force generation, and contractile protein expression and myofibrillar structure. Since hESC-derived cardiomyocytes have the potential to engraft into surgical models of heart disease, they have been considered for cardiomyocyte replacement therapy and as well as a tool to discover drugs capable of stimulating endogenous regeneration. Despite such encouraging advances, the application of hESCderived cardiomyocytes for basic developmental research and large-scale applications, such as high throughput screening, toxicology testing and large animal studies, has been hindered by their poor yield from the heterogeneous hESC cultures and the difficulty of manipulating hESCs to express uniform levels of reporter constructs. We have therefore developed methods and vectors to produce homogeneous hESC lines with fluorescent and drug-selectable markers that permit isolation of pure populations of labeled stem cells and hESC-derived 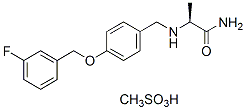 cardiomyocytes. Prior strategies to increase the yield of cardiomyocytes from hESCs have included optimizing culture regimens by the addition of growth factors and other reagents to direct differentiation. Although such advances quantitatively improved the proportion of the cells that differentiate into cardiomyocytes, in most settings the yield remains between 5�C25%. Strategies for enrichment have included manual dissection of beating areas, PercollH density gradient sedimentation, and fluorescence activated cell sorting of cells based on expression of a fluorescent reporter protein from cardiomyocyte gene Ginsenoside-F4 promoters. Each of these strategies has drawbacks in terms of purity, viability and scalability. In theory, an effective alternative is drug resistance based selection of cardiomyocytes as successfully implemented by Field and collaborators using the Neomycin analogue G418 to purify cardiomyocytes from differentiating mouse ESC cultures. Genetic selection has recently been adapted to hESCs, and we applied this technology with a suite of lentiviral vectors and protocols for the production of Diperodon stable.
cardiomyocytes. Prior strategies to increase the yield of cardiomyocytes from hESCs have included optimizing culture regimens by the addition of growth factors and other reagents to direct differentiation. Although such advances quantitatively improved the proportion of the cells that differentiate into cardiomyocytes, in most settings the yield remains between 5�C25%. Strategies for enrichment have included manual dissection of beating areas, PercollH density gradient sedimentation, and fluorescence activated cell sorting of cells based on expression of a fluorescent reporter protein from cardiomyocyte gene Ginsenoside-F4 promoters. Each of these strategies has drawbacks in terms of purity, viability and scalability. In theory, an effective alternative is drug resistance based selection of cardiomyocytes as successfully implemented by Field and collaborators using the Neomycin analogue G418 to purify cardiomyocytes from differentiating mouse ESC cultures. Genetic selection has recently been adapted to hESCs, and we applied this technology with a suite of lentiviral vectors and protocols for the production of Diperodon stable.