Similarly, our study highlights perturbations in the relative abundance of several caveolar proteins in FSHD myotubes. A defect in these membrane microdomain subtypes could also contribute to myotube 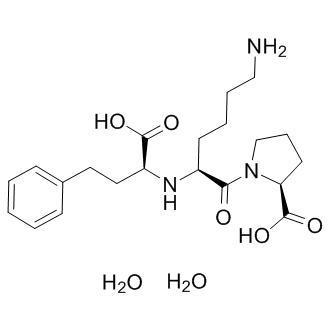 deformation in FSHD. Because the alteration of caveolar proteins was also found in atrophic myotubes, further studies are necessary to precisely determine the contribution of each factor to the formation of a given phenotype. Because the predominantly atrophic or disorganized FSHD cultures that we have Albaspidin-AA analyzed are derived from comparable patients in terms of the number of D4Z4 units, sex and age, we assume that other factors could intervene to explain the emergence of a non-atrophic phenotype, despite the expression of DUX4. Other genes were suggested to be involved in FSHD, including FRG1, ANT1 and DUX4c, but further studies are necessary to explain the relative contribution of each 4q35 gene in FSHD. DUX4c is induced in FSHD muscles and could bind to DUX4-target promoters through its identical double homeodomain, as was described for PITX1. Because DUX4c overexpression is associated with increased myoblast proliferation and decreased differentiation, it is a good candidate to explain the emergence of a non-atrophic phenotype. DUX4-s, which is a putative protein derived from a short DUX4 mRNA variant that is often detected in control muscles and less frequently in FSHD muscles, was suggested to act as a dominant negative variant. DUX4-s may also take part in this process, but further studies are needed to determine whether this protein is endogenously expressed in FSHD or control muscle cells. The present data indicate that FSHD myotubes present clear changes in the relative abundance of proteins typical of caveolae, which are membrane lipid microdomains that are enriched in cholesterol and glycosphingolipids and are often considered to be a specialized lipid raft subtype. These membrane invaginations play a major role in signal transduction and appear to constitute signaling platforms that mediate the sequestration of certain receptors, transporters and signaling proteins. They are thus involved in numerous biological processes, e.g., membrane repair, redox signaling, immune response and lipid metabolism. Caveolin3 is the main caveolar protein in skeletal muscle and is a key factor in muscle cell fusion, and several mutations in the CAV3 gene cause heterogeneous neuromuscular diseases including caveolinopathies such as LGMD1. Caveolin-associated cavins, particularly PTRF/cavin-1, are crucial regulators of caveola formation. MURC/cavin-4 was first described as a cytosolic protein that is partly localized in the Z-line and associated with cardiac dysfunction through the modulation of the Rho/ROCK pathway. MURC expression is increased Lomitapide Mesylate during the differentiation of C2C12 myoblasts, and its RNAi-mediated knockdown impairs myogenic differentiation. In the present study, we reported a decreased level of MURC in FSHD myotubes. FSHD myoblasts fail to upregulate MURC during their differentiation, and this perturbation could also be linked to the general dampening of myogenic differentiation associated with FSHD as described in. Recently, MURC was found to be localized to sarcolemmal caveolae in normal muscle, with an impaired distribution in muscle from a patient with heterogeneous CAV3 expression, which suggests a potential role of MURC in caveolin-associated muscle disease. In conclusion, the use of an optimized proteomic approach has enabled us to define molecular differences between atrophic and disorganized FSHD.
deformation in FSHD. Because the alteration of caveolar proteins was also found in atrophic myotubes, further studies are necessary to precisely determine the contribution of each factor to the formation of a given phenotype. Because the predominantly atrophic or disorganized FSHD cultures that we have Albaspidin-AA analyzed are derived from comparable patients in terms of the number of D4Z4 units, sex and age, we assume that other factors could intervene to explain the emergence of a non-atrophic phenotype, despite the expression of DUX4. Other genes were suggested to be involved in FSHD, including FRG1, ANT1 and DUX4c, but further studies are necessary to explain the relative contribution of each 4q35 gene in FSHD. DUX4c is induced in FSHD muscles and could bind to DUX4-target promoters through its identical double homeodomain, as was described for PITX1. Because DUX4c overexpression is associated with increased myoblast proliferation and decreased differentiation, it is a good candidate to explain the emergence of a non-atrophic phenotype. DUX4-s, which is a putative protein derived from a short DUX4 mRNA variant that is often detected in control muscles and less frequently in FSHD muscles, was suggested to act as a dominant negative variant. DUX4-s may also take part in this process, but further studies are needed to determine whether this protein is endogenously expressed in FSHD or control muscle cells. The present data indicate that FSHD myotubes present clear changes in the relative abundance of proteins typical of caveolae, which are membrane lipid microdomains that are enriched in cholesterol and glycosphingolipids and are often considered to be a specialized lipid raft subtype. These membrane invaginations play a major role in signal transduction and appear to constitute signaling platforms that mediate the sequestration of certain receptors, transporters and signaling proteins. They are thus involved in numerous biological processes, e.g., membrane repair, redox signaling, immune response and lipid metabolism. Caveolin3 is the main caveolar protein in skeletal muscle and is a key factor in muscle cell fusion, and several mutations in the CAV3 gene cause heterogeneous neuromuscular diseases including caveolinopathies such as LGMD1. Caveolin-associated cavins, particularly PTRF/cavin-1, are crucial regulators of caveola formation. MURC/cavin-4 was first described as a cytosolic protein that is partly localized in the Z-line and associated with cardiac dysfunction through the modulation of the Rho/ROCK pathway. MURC expression is increased Lomitapide Mesylate during the differentiation of C2C12 myoblasts, and its RNAi-mediated knockdown impairs myogenic differentiation. In the present study, we reported a decreased level of MURC in FSHD myotubes. FSHD myoblasts fail to upregulate MURC during their differentiation, and this perturbation could also be linked to the general dampening of myogenic differentiation associated with FSHD as described in. Recently, MURC was found to be localized to sarcolemmal caveolae in normal muscle, with an impaired distribution in muscle from a patient with heterogeneous CAV3 expression, which suggests a potential role of MURC in caveolin-associated muscle disease. In conclusion, the use of an optimized proteomic approach has enabled us to define molecular differences between atrophic and disorganized FSHD.