A value also detected in parental and control cells stably transfected with empty vector, that was close to that of HspB5. 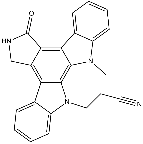 Moreover, the constitutive expression of wild type or mutant HspB5 did not modify the level of Hsp90, Hsp70, and HspB1, hence suggesting that the presence of exogenous HspB5 polypeptides inside HeLa cells was not sensed as a stress. We also tested for the presence of HspB6, a member of the family of small Hsps that can form chimeric hetero-oligomers with HspB1 in vitro. This protein was barely detectable in Neo, WT and R120G cells and was therefore not further studied. HspB1 and HspB5 appear therefore as the major interacting small Hsps that are present in Neo, WT and R120G cells. We next analyzed of the effects mediated by their interaction inside cells. Similar conclusions could be drawn when the antibody targeting HspB5 was used to perform IP. Aliquots of the total and immunodepleted pooled fractions were analyzed to verify if the immunoprecitation of the targeted protein was complete and to test the fate of the other protein partner. Since the immunodepleted supernatant fractions were devoid of the corresponding proteins, it was concluded that 100% of both HspB1 and HspB5 present in the pooled fractions were interacting and formed chimeric complexes. We then tested HspB1 and HspB5 interaction in presence of 300 mM NaCl. As seen in Fig. 2Db, interaction between HspB1 and HspB5 in the pooled fractions from WT cells was weakened by the 300 mM wash. Indeed, in the presence of high salt, a fraction of HspB5 or HspB1 corresponding partner was removed from the complex and recovered in the immunodepleted supernatant. The VE-822 in vivo phenomenon was not observed when a similar analysis was performed using the pooled fractions from R120G cells. In this case the interaction was not altered by 300 mM NaCl, hence suggesting that it was tightened by the R120G mutation. We next analyzed the resistance of Neo, WT and R120G cells to oxidative conditions since this is a common stress encountered by cells expressing HspB5, as for example when they are exposed to UV light or chronic inflammation damages. Neo, WT and R120G cells were exposed for different time periods to 60 or 100 mM of menadione, a compound that generates intracellular reactive oxygen species via redoxcycling. Subsequently, their survival was determined using crystal violet staining, clonogenic colony formation assay and phase-contrast analysis of live cells. It is seen in Fig. 3A,B, that WT cells were significantly more oxidoresistant than Neo cells while R120G cells displayed a pronounced sensitivity to menadione. Similar observations were made using Trypan blue staining of dead cells and after exposure to 100 mM of hydrogen peroxide. Morphological analysis, presented in Fig. 3C, revealed the accumulation of perinuclear vacuoles and granules in menadione-treated Neo cells that were not detected in WT cells. However, in spite of their apparent resistance to menadione, WT cells no more displayed an elongated morphology and had a more polygonal morphology; a phenomenon that could be related to the sensitivity of F-actin cytoskeleton to menadione induced oxidative stress. About half of menadione-treated R120G cells had a dying morphology: they were detached from the substratum, had a rounded appearance and were linked to each other by filamentous PF-4217903 bridges. The remaining living R120G cells were still attached and displayed a polygonal appearance. However, they had lost their dense membranous ruffles in the leading edges and were loaded with vacuoles and granules. Control experiments revealed no changes in the cellular content of HspB1 and HspB5 in response of menadione treatment as well as no stimulation of the level of two major ATP-dependent chaperones, Hsp70 and Hsp90.
Moreover, the constitutive expression of wild type or mutant HspB5 did not modify the level of Hsp90, Hsp70, and HspB1, hence suggesting that the presence of exogenous HspB5 polypeptides inside HeLa cells was not sensed as a stress. We also tested for the presence of HspB6, a member of the family of small Hsps that can form chimeric hetero-oligomers with HspB1 in vitro. This protein was barely detectable in Neo, WT and R120G cells and was therefore not further studied. HspB1 and HspB5 appear therefore as the major interacting small Hsps that are present in Neo, WT and R120G cells. We next analyzed of the effects mediated by their interaction inside cells. Similar conclusions could be drawn when the antibody targeting HspB5 was used to perform IP. Aliquots of the total and immunodepleted pooled fractions were analyzed to verify if the immunoprecitation of the targeted protein was complete and to test the fate of the other protein partner. Since the immunodepleted supernatant fractions were devoid of the corresponding proteins, it was concluded that 100% of both HspB1 and HspB5 present in the pooled fractions were interacting and formed chimeric complexes. We then tested HspB1 and HspB5 interaction in presence of 300 mM NaCl. As seen in Fig. 2Db, interaction between HspB1 and HspB5 in the pooled fractions from WT cells was weakened by the 300 mM wash. Indeed, in the presence of high salt, a fraction of HspB5 or HspB1 corresponding partner was removed from the complex and recovered in the immunodepleted supernatant. The VE-822 in vivo phenomenon was not observed when a similar analysis was performed using the pooled fractions from R120G cells. In this case the interaction was not altered by 300 mM NaCl, hence suggesting that it was tightened by the R120G mutation. We next analyzed the resistance of Neo, WT and R120G cells to oxidative conditions since this is a common stress encountered by cells expressing HspB5, as for example when they are exposed to UV light or chronic inflammation damages. Neo, WT and R120G cells were exposed for different time periods to 60 or 100 mM of menadione, a compound that generates intracellular reactive oxygen species via redoxcycling. Subsequently, their survival was determined using crystal violet staining, clonogenic colony formation assay and phase-contrast analysis of live cells. It is seen in Fig. 3A,B, that WT cells were significantly more oxidoresistant than Neo cells while R120G cells displayed a pronounced sensitivity to menadione. Similar observations were made using Trypan blue staining of dead cells and after exposure to 100 mM of hydrogen peroxide. Morphological analysis, presented in Fig. 3C, revealed the accumulation of perinuclear vacuoles and granules in menadione-treated Neo cells that were not detected in WT cells. However, in spite of their apparent resistance to menadione, WT cells no more displayed an elongated morphology and had a more polygonal morphology; a phenomenon that could be related to the sensitivity of F-actin cytoskeleton to menadione induced oxidative stress. About half of menadione-treated R120G cells had a dying morphology: they were detached from the substratum, had a rounded appearance and were linked to each other by filamentous PF-4217903 bridges. The remaining living R120G cells were still attached and displayed a polygonal appearance. However, they had lost their dense membranous ruffles in the leading edges and were loaded with vacuoles and granules. Control experiments revealed no changes in the cellular content of HspB1 and HspB5 in response of menadione treatment as well as no stimulation of the level of two major ATP-dependent chaperones, Hsp70 and Hsp90.