Compared to SP1 the peptides from group II and III lack the positively charged N-terminus. This feature might favour the “peptide-pathogen” interaction resulting in an inhibition of bacterial multiplication. The findings of Alvarez-Bravo and coworkers support this conclusion. Their experiments are based on synthetic peptides reflecting the antimicrobial active core of sapecin B, an insect AMP found in the larval INCB28060 hemolymph of the flesh fly. They generated several short peptides mainly composed of a stretch of leucine residues forming the hydrophobic core bordered by lysine and arginine containing sequences at the termini. For their activity a terminal KLK or RLK motif was critical. In addition, the length of the hydrophobic 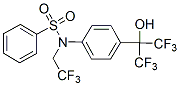 core was important for the antibacterial activity but, surprisingly, not for the antifungal properties of the peptides. Hence, beside the positive net-charge also terminal structures of the peptides might contribute to the XL880 extent of their antimicrobial properties. Within the peptides of group IV only SP13 and SP16 were active against the tested bacterial microorganisms. Especially SP13 showed similar activity against C. michiganensis, X. vesicatoria, and P. corrugata as SP1 and SP2 with a MIC of 2.5 mg/ml. Interestingly, in SP13 as well as in SP16 the charged terminal parts are connected via three charged amino acids. Probably this structural characteristic allows a defined pore-like incorporation into the bacterial membrane. Interestingly, the peptides containing D-amino acids showed significant lower low hemolytic activity than the corresponding Lforms. This was also observed in previous studies for other peptides. Furthermore, these peptides are more active against fungal pathogens suggesting an increased resistance against degradation. Most of the previous work on AMP is based on in-vitro data demonstrating the activity of the peptides in growth media. However, in-vitro and in-vivo conditions differ greatly and results obtained from in-vitro inhibition assays may just serve as indication for the potency of compounds in-vivo. In the plant microenvironment complex factors might affect the interaction between peptides and pathogens. Therefore, inhibition studies on susceptible plant tissue have to be carried out to analyse the potential use of the peptides for plant protection. Most assays are based on detached leaves or leaf disks, flowers or fruits. With such assays it is possible to investigate the activity of the peptides on the plant surface, but the way they are performed did not reflect a potential way of practical application. In nature bacteria are spread by wind and rain, penetrate leaves and fruits through stomata and wounds and multiply intercellular to induce lesions on stems, leaves and especially fruits. To simulate the natural inoculation, bacteria were sprayed onto the tomato leaf surface. Using this spraying technique, we could demonstrate that peptides SP1-1, SP10-2 and SP10-5 successfully inhibited the proliferation of P. syringae pv. tomato on tomato leaves. The higher peptide concentrations needed in the spraying assays is probably due to the higher amount of bacteria used to infect plants. Furthermore, degradation on the plant surface cannot be excluded. Especially after wounding the antimicrobial activities of the peptides are reduced. At such areas apoplast and/or tissue fluids are leaking and in such fluids the AMPs may be inactivated by protease-based degradation or by binding or reacting with phenolic compounds. Interestingly, the peptides SP1-1 and SP10-5 are significantly more resistant against apoplast fluid dependent inactivation than SP10-2. Moreover, SP10-5 is inhibiting symptom development after injection into tomato fruits. These results make peptide SP10-5 a promising candidate for transgenic approaches.
core was important for the antibacterial activity but, surprisingly, not for the antifungal properties of the peptides. Hence, beside the positive net-charge also terminal structures of the peptides might contribute to the XL880 extent of their antimicrobial properties. Within the peptides of group IV only SP13 and SP16 were active against the tested bacterial microorganisms. Especially SP13 showed similar activity against C. michiganensis, X. vesicatoria, and P. corrugata as SP1 and SP2 with a MIC of 2.5 mg/ml. Interestingly, in SP13 as well as in SP16 the charged terminal parts are connected via three charged amino acids. Probably this structural characteristic allows a defined pore-like incorporation into the bacterial membrane. Interestingly, the peptides containing D-amino acids showed significant lower low hemolytic activity than the corresponding Lforms. This was also observed in previous studies for other peptides. Furthermore, these peptides are more active against fungal pathogens suggesting an increased resistance against degradation. Most of the previous work on AMP is based on in-vitro data demonstrating the activity of the peptides in growth media. However, in-vitro and in-vivo conditions differ greatly and results obtained from in-vitro inhibition assays may just serve as indication for the potency of compounds in-vivo. In the plant microenvironment complex factors might affect the interaction between peptides and pathogens. Therefore, inhibition studies on susceptible plant tissue have to be carried out to analyse the potential use of the peptides for plant protection. Most assays are based on detached leaves or leaf disks, flowers or fruits. With such assays it is possible to investigate the activity of the peptides on the plant surface, but the way they are performed did not reflect a potential way of practical application. In nature bacteria are spread by wind and rain, penetrate leaves and fruits through stomata and wounds and multiply intercellular to induce lesions on stems, leaves and especially fruits. To simulate the natural inoculation, bacteria were sprayed onto the tomato leaf surface. Using this spraying technique, we could demonstrate that peptides SP1-1, SP10-2 and SP10-5 successfully inhibited the proliferation of P. syringae pv. tomato on tomato leaves. The higher peptide concentrations needed in the spraying assays is probably due to the higher amount of bacteria used to infect plants. Furthermore, degradation on the plant surface cannot be excluded. Especially after wounding the antimicrobial activities of the peptides are reduced. At such areas apoplast and/or tissue fluids are leaking and in such fluids the AMPs may be inactivated by protease-based degradation or by binding or reacting with phenolic compounds. Interestingly, the peptides SP1-1 and SP10-5 are significantly more resistant against apoplast fluid dependent inactivation than SP10-2. Moreover, SP10-5 is inhibiting symptom development after injection into tomato fruits. These results make peptide SP10-5 a promising candidate for transgenic approaches.