Therefore, genetic expression and molecular functions of CD36 are complex and controlled by membrane and tissue specific molecular associations and different cellular specific signaling pathways. This pleiotropic effect may reasonably well question the clinical relevance and safety of CD36. While the cellular functions of CD36 are recognized, its importance in the physiopathology is less well understood and often controversial. The role of CD36 in the formation of foam cells and the growth of atherosclerotic plaques is well documented. Yet the role of CD36 as a target to combat atherosclerosis was criticized. Similarly, evidences supporting a role of CD36 in intestinal fat absorption are accumulated, but contradictory observations have also been reported concerning its direct implication in intestinal lipid trafficking and the control of postprandial hypertriglyceridemia. For instance, CD36 is expressed all through the intestinal tract and is important for the metabolism and the secretion of chylomicron into the lymph. The molecule is required for efficient intestinal absorption of LCFA and VLCFA. Yet, CD36 deficient mice exhibit a normal level of FA absorption and gene deletion does not affect LCFA uptake and TG re-esterification in mouse jejunum. Therefore the potential of CD36 as a therapeutic target is debated. In the present paper we have identified small chemical molecules which have the capacity to inhibit the FA and ox-LDL receptor function of CD36. These inhibitors were able to rescue well characterized animal models from 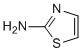 postprandial hypertriglyceridemia and atherosclerosis with a concomitant improvement of insulin resistance and Ibrutinib glucose tolerance. The CD36-inhibitor activity of this new chemical series was established on the following criteria. First, the molecules were selected for their capacity to inhibit ox-LDL binding, uptake and accumulation in THP1 cells. Furthermore, using CD36-transfected HEK cells the specificity of this inhibition for CD36 was demonstrated. Active members of this series were able to completely inhibit GDC-0941 binding and uptake to levels that were similar to the non-specific binding and uptake observed with wt cells. Second, consistent with the dual function of CD36 as a receptor for two different ligands, and the non-competitive agonist activity of these inhibitors, a similar activity on LCFA binding and uptake on both THP1 and HEK-CD36 cells was measured. These results support a receptor rather than a ligand-driven inhibition. Third, analogs of the same series with close chemical structure had no effect on these cellular functions, suggesting the existence of a structure-function relationship within the members of the series. Finally, cross-linking affinity was used to demonstrate the effect of the compounds on the molecular interaction between ox-LDL and CD36. In aggregate, these new molecules were able to inhibit the CD36 receptor function both at the cellular and the molecular levels. The first CD36 in vivo activity to be examined was its implication in the development of atherosclerosis using a well characterized animal model. A DKO mouse combining LDL-R and leptin deficiencies was used. This model exhibits high blood pressure together with increased plasma TG concentration, insulin and glucose. It develops atherosclerosis and represents a good model to study the physiopathology of the metabolic syndrome. The CD36-antagonists used in the present study were able to reduce the growth of atherosclerotic plaques at plasma concentrations compatible with the cellular activity of these molecules. This is in agreement with the fact that CD36 depleted mice are protected against atherosclerosis. Unexpectedly, a significant reduction of the plasma TG was also observed.
postprandial hypertriglyceridemia and atherosclerosis with a concomitant improvement of insulin resistance and Ibrutinib glucose tolerance. The CD36-inhibitor activity of this new chemical series was established on the following criteria. First, the molecules were selected for their capacity to inhibit ox-LDL binding, uptake and accumulation in THP1 cells. Furthermore, using CD36-transfected HEK cells the specificity of this inhibition for CD36 was demonstrated. Active members of this series were able to completely inhibit GDC-0941 binding and uptake to levels that were similar to the non-specific binding and uptake observed with wt cells. Second, consistent with the dual function of CD36 as a receptor for two different ligands, and the non-competitive agonist activity of these inhibitors, a similar activity on LCFA binding and uptake on both THP1 and HEK-CD36 cells was measured. These results support a receptor rather than a ligand-driven inhibition. Third, analogs of the same series with close chemical structure had no effect on these cellular functions, suggesting the existence of a structure-function relationship within the members of the series. Finally, cross-linking affinity was used to demonstrate the effect of the compounds on the molecular interaction between ox-LDL and CD36. In aggregate, these new molecules were able to inhibit the CD36 receptor function both at the cellular and the molecular levels. The first CD36 in vivo activity to be examined was its implication in the development of atherosclerosis using a well characterized animal model. A DKO mouse combining LDL-R and leptin deficiencies was used. This model exhibits high blood pressure together with increased plasma TG concentration, insulin and glucose. It develops atherosclerosis and represents a good model to study the physiopathology of the metabolic syndrome. The CD36-antagonists used in the present study were able to reduce the growth of atherosclerotic plaques at plasma concentrations compatible with the cellular activity of these molecules. This is in agreement with the fact that CD36 depleted mice are protected against atherosclerosis. Unexpectedly, a significant reduction of the plasma TG was also observed.