In vivo, pericytes have been shown to differentiate into smooth muscle cells and myofibroblasts, and in vitro to osteoblasts, adipocytes and chondroblast. Based on studies on human pathological conditions and animal models, we and others have proposed that pericytes in inflammatory conditions including wound healing in adult tissues become activated and expand into pro-fibrotic connective tissue cells, indicating that these microvascular cells play a central role in tissue fibrosis. We have developed methods to isolate and propagate pericytes from placenta and neonatal skin which has enabled the study of AB1010 pericyte biology and in particular their differentiation into collagen type I producing fibroblasts, a process that spontaneously takes place when pericytes are cultured in the presence of 10% fetal calf serum. Epigenetic inheritance represents heritable patterns in gene expression caused by mechanisms other than changes in underlying DNA sequences. Thus, non-genetic factors can cause genes to behave differently opening up possibilities such as modifying 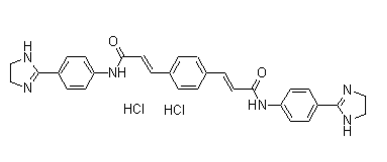 the phenotype of congenital and acquired diseases. Furthermore, epigenetic mechanisms are believed to modify the progeny of stem/ progenitor cells as well as affect the phenotypes of individual specialized cells and maintain these phenotypes over several rounds of cell division thus constituting a mechanism by which cells have “memory”. The molecular basis of epigenetics is complex but includes DNA methylation and post-translational modifications of histones such as acetylation and methylation. These DNA and histone modifications affect chromatin structure, thereby regulating gene expression. The state of histone acetylation is a balance between reciprocal enzymes, histone acetyltransferases and histone deactetylases, acetylating and deacetylating histones, TWS119 respectively. Acetylation generally leads to transcriptionally active chromatin while deacetylation results in transcriptional silencing. Valproic acid is an aliphatic acid compound that has been used for decades as a medication to treat epilepsy and as a mood stabilizer in bipolar disorder. VPA is one among several HDAC inhibitors. VPA is also recognized for its teratogenic effects in humans. HDAC inhibition results in hyperacetylation of histones and affects gene expression, which in turn influences cellular proliferation, differentiation, apoptosis and migration as well as more complex multi-cellular processes such as angiogenesis and fibrosis. Thus, HDAC inhibition has therefore been postulated to affect genetic perturbations as occurs in for instance congenital and neoplastic disease. Studies have shown potential beneficial effects of HDAC inhibition in a wide array of diseases including cystic fibrosis, malignancies, myelofibrosis and neurodegenerative diseases such as Alzheimers and Huntingtons disease. Several in vitro and in vivo studies have shown that HDAC inhibition is anti-angiogenic. The present in vitro study is the first investigation into the biological consequences of HDAC inhibition in well-defined primary human microvascular pericytes. HDAC inhibition reduced pericyte proliferation and inhibition of migration without significantly affecting cell viability. The differentiation of pericytes into pro-fibrotic connective tissue cells was reduced by VPA suggesting a novel pericyte based process of how HDAC inhibition may perturb fibrosis. Furthermore, a limited qPCR microarray approach focusing on mRNAs coding for proteins involved in angiogenesis was performed in freshly isolated human pericytes revealing expression patterns of angiogenic mRNAs and the potential effect.
the phenotype of congenital and acquired diseases. Furthermore, epigenetic mechanisms are believed to modify the progeny of stem/ progenitor cells as well as affect the phenotypes of individual specialized cells and maintain these phenotypes over several rounds of cell division thus constituting a mechanism by which cells have “memory”. The molecular basis of epigenetics is complex but includes DNA methylation and post-translational modifications of histones such as acetylation and methylation. These DNA and histone modifications affect chromatin structure, thereby regulating gene expression. The state of histone acetylation is a balance between reciprocal enzymes, histone acetyltransferases and histone deactetylases, acetylating and deacetylating histones, TWS119 respectively. Acetylation generally leads to transcriptionally active chromatin while deacetylation results in transcriptional silencing. Valproic acid is an aliphatic acid compound that has been used for decades as a medication to treat epilepsy and as a mood stabilizer in bipolar disorder. VPA is one among several HDAC inhibitors. VPA is also recognized for its teratogenic effects in humans. HDAC inhibition results in hyperacetylation of histones and affects gene expression, which in turn influences cellular proliferation, differentiation, apoptosis and migration as well as more complex multi-cellular processes such as angiogenesis and fibrosis. Thus, HDAC inhibition has therefore been postulated to affect genetic perturbations as occurs in for instance congenital and neoplastic disease. Studies have shown potential beneficial effects of HDAC inhibition in a wide array of diseases including cystic fibrosis, malignancies, myelofibrosis and neurodegenerative diseases such as Alzheimers and Huntingtons disease. Several in vitro and in vivo studies have shown that HDAC inhibition is anti-angiogenic. The present in vitro study is the first investigation into the biological consequences of HDAC inhibition in well-defined primary human microvascular pericytes. HDAC inhibition reduced pericyte proliferation and inhibition of migration without significantly affecting cell viability. The differentiation of pericytes into pro-fibrotic connective tissue cells was reduced by VPA suggesting a novel pericyte based process of how HDAC inhibition may perturb fibrosis. Furthermore, a limited qPCR microarray approach focusing on mRNAs coding for proteins involved in angiogenesis was performed in freshly isolated human pericytes revealing expression patterns of angiogenic mRNAs and the potential effect.