These kinases in the human kinome belong to a unique and isolated subfamily with only three proteins VRK that very early, and near the kinases common trunk, diverged from the branch that much later led to casein kinase I family. In addition, the VRK proteins have unique substitutions suggesting they might be pseudokinases. VRK1 and VRK2 are two novel Ser-Thr kinases that have a common catalytic domain with a fifty-three percent homology, and play a role in cell division processes. However, VRK1 and VRK2 have been demonstrated to be catalytically active; while VRK3, the most divergent of the three, is catalytically inactive. Interestingly, the kinase activity of VRK1 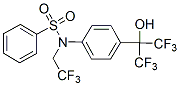 and VRK2 proteins can be regulated by allosteric protein-protein interactions; they are both kinase active when bound to RanGTP, and kinase-inactive when bound to RanGDP. This indicates that these two kinases have two alternative conformations that can be allosterically regulated. VRK1 is a nuclear kinase, while VRK2 has two isoforms, a full-length protein of 508 aminoacids, which is anchored to cytosolic organelle membranes, such as endoplasmic reticulum and mitochondria by its Cterminal hydrophobic anchoring region; and VRK2B, with 397 aminoacids lacking the C-terminal region and detected both in cytosol and nucleus, perhaps functionally replacing in some aspects VRK1 and detected only in some INCB28060 cellular types, like adenocarcinomas. The conservation in catalytic domain and different subcellular location indicate that substrate utilization, and perhaps specificity, might determine signal compartmentalization and substrate use. The regulation of kinases in time and space is likely to be an area of intense research in the future. VRK1 is expressed at high levels in tumours with p53 mutations, such as in lung cancer and identifies a subgroup of breast cancer with a poorer prognosis. VRK1 is the best characterized protein of the VRK family regarding its substrates, that include Foretinib phosphorylation of p53 in T18, c-Jun in S63 and S73, ATF2 in Ser62 and T73, CREB1 in S133 and histone H3 in T3 and S10, this latter modification regulates methylation and affects chromatin structure. Also, VRK1 functions as a coordinator of several processes required for cell division, identifies a bad prognosis signature in breast cancer, and specific expression patterns in human tissues, normal and malignant. Kinase inhibitor screenings have not yet identified any inhibitor for the VRK family, consistent with its low promiscuity index. Kinases can be discriminated using a small panel of thirty-eight inhibitors and three hundred and seventeen kinases as targets, including both tyrosine and serine-threonine kinases. The atypical structure of VRK proteins determined by specific aminoacid substitutions makes them suitable targets for development of specific inhibitors with reduced kinase promiscuity. Therefore, in this work we have aimed to determine if catalytically active VRK1 and VRK2 proteins have similar or different sensitivity to current kinase inhibitors with the aim to obtain the starting point for future development of kinase specific inhibitors with limited or no cross-inhibition. One of the main implications of VRK proteins is their potential utilization for developing specific inhibitors that may be used in oncologic treatments. But a main problem with current inhibitors is that they usually affect several related kinases simultaneously, although there might be some differences in affinity. In practice, this means that the clinical use of inhibitors affecting several kinases might present a significant risk of uncontrolled side effects.
and VRK2 proteins can be regulated by allosteric protein-protein interactions; they are both kinase active when bound to RanGTP, and kinase-inactive when bound to RanGDP. This indicates that these two kinases have two alternative conformations that can be allosterically regulated. VRK1 is a nuclear kinase, while VRK2 has two isoforms, a full-length protein of 508 aminoacids, which is anchored to cytosolic organelle membranes, such as endoplasmic reticulum and mitochondria by its Cterminal hydrophobic anchoring region; and VRK2B, with 397 aminoacids lacking the C-terminal region and detected both in cytosol and nucleus, perhaps functionally replacing in some aspects VRK1 and detected only in some INCB28060 cellular types, like adenocarcinomas. The conservation in catalytic domain and different subcellular location indicate that substrate utilization, and perhaps specificity, might determine signal compartmentalization and substrate use. The regulation of kinases in time and space is likely to be an area of intense research in the future. VRK1 is expressed at high levels in tumours with p53 mutations, such as in lung cancer and identifies a subgroup of breast cancer with a poorer prognosis. VRK1 is the best characterized protein of the VRK family regarding its substrates, that include Foretinib phosphorylation of p53 in T18, c-Jun in S63 and S73, ATF2 in Ser62 and T73, CREB1 in S133 and histone H3 in T3 and S10, this latter modification regulates methylation and affects chromatin structure. Also, VRK1 functions as a coordinator of several processes required for cell division, identifies a bad prognosis signature in breast cancer, and specific expression patterns in human tissues, normal and malignant. Kinase inhibitor screenings have not yet identified any inhibitor for the VRK family, consistent with its low promiscuity index. Kinases can be discriminated using a small panel of thirty-eight inhibitors and three hundred and seventeen kinases as targets, including both tyrosine and serine-threonine kinases. The atypical structure of VRK proteins determined by specific aminoacid substitutions makes them suitable targets for development of specific inhibitors with reduced kinase promiscuity. Therefore, in this work we have aimed to determine if catalytically active VRK1 and VRK2 proteins have similar or different sensitivity to current kinase inhibitors with the aim to obtain the starting point for future development of kinase specific inhibitors with limited or no cross-inhibition. One of the main implications of VRK proteins is their potential utilization for developing specific inhibitors that may be used in oncologic treatments. But a main problem with current inhibitors is that they usually affect several related kinases simultaneously, although there might be some differences in affinity. In practice, this means that the clinical use of inhibitors affecting several kinases might present a significant risk of uncontrolled side effects.