The overall collagen content was assessed by measuring the orthohydroxyprolinecontent via dimethylaminobenzaldehyde and chloramine T assay. Collagen content was 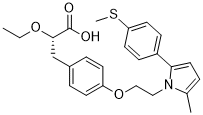 calculated by assuming a 1:7.5 OHP-to-collagen mass ratio. The collagen and GAG contents were normalized to the disk wet weight. Calcium content of the samples was analyzed using a calcium quantification kit. In this study, an in vitro hypertrophy model was used to evaluate hMSC hypertrophy after Lithium citrate chondrogenic induction. This model allows us to examine the influence of cell-mediated degradation on hMSC hypertrophy in a defined in vitro setting, thereby avoiding the systemic complexity and considerable expense of in vivo studies. Switching to the hypertrophy induction media after 2 weeks of chondrogenic induction not only expedites the hypertrophic differentiation of hMSCs by eliminating the hypertrophy-suppressing TGF-b3 but also provides the necessary phosphate donors for mineralization. Therefore, several previous studies employed this in vitro model to study hMSC hypertrophy. It is known that hydrogels Chloroquine Phosphate fabricated using methacrylated hyaluronic acidexperience little hydrolytic degradation over extended period likely due to the methyl groups in the methacrylates, which shield water molecules by steric hindrance. Our previous study indicated that high crosslinking density of MeHA hydrogels resulted in reduced cartilage matrix biosynthesis and more restricted cartilage matrix distribution. The hypertrophic differentiation of the hMSCs and ensuing neocartilage calcification was also enhanced in highly crosslinked MeHA hydrogels compared to hydrogels of low crosslinking density. Prior studies showed that MMP-sensitive PEG hydrogels promoted neocartilage development by the encapsulated chondrocytes and MSCs. Compared with PEG hydrogels, HA hydrogels afford a more favorable microenvironment for chondrogenesis by providing binding sites for cell surface receptors such as CD44 and CD168, activation of which are important to chondrogenesis. This study confirmed that MMP-sensitive HA hydrogels also enhanced chondrogenesis of the encapsulated hMSCs. The enhanced cell spreading and motility in the MMP-sensitive hydrogels allowed more cell-cell interactions, which are essential to chondrogenesis. The proteolytic action of MMPs also releases ECM bound growth factors that are inducer of chondrogenesis. These may have contributed to the enhanced chondrogenesis of the hMSCs in the MMP-sensitive HA hydrogels. Importantly, prior design of MMP-sensitivity in HA hydrogels was achieved by crosslinking acrylated HA with crosslinkers containing dual thiols. The resulting thiol-ether-ester bonds are potentially labile to hydrolytic degradation. A separate unpublished study revealed that the acrylated HAhydrogels quickly degraded and lost the structural integrity within a week of culture. This not only makes the acrylated HA hydrogel unsuitable for long term cell culture studies but also confounds the effect of MMP-mediated degradation on hMSCs. In contrast, maleimides react with thiols to form stable thioether bonds. A prior study demonstrated that maleimide modified HAmacromers can be crosslinked with the same thiol containing crosslinkers to form hydrogelsthat are resistant to hydrolytic degradation. In contrast to the acrylated HA hydrogels used in this study largely maintained their initial cylindrical morphology during the 4 weeks of culture without significant bulk degradation.
calculated by assuming a 1:7.5 OHP-to-collagen mass ratio. The collagen and GAG contents were normalized to the disk wet weight. Calcium content of the samples was analyzed using a calcium quantification kit. In this study, an in vitro hypertrophy model was used to evaluate hMSC hypertrophy after Lithium citrate chondrogenic induction. This model allows us to examine the influence of cell-mediated degradation on hMSC hypertrophy in a defined in vitro setting, thereby avoiding the systemic complexity and considerable expense of in vivo studies. Switching to the hypertrophy induction media after 2 weeks of chondrogenic induction not only expedites the hypertrophic differentiation of hMSCs by eliminating the hypertrophy-suppressing TGF-b3 but also provides the necessary phosphate donors for mineralization. Therefore, several previous studies employed this in vitro model to study hMSC hypertrophy. It is known that hydrogels Chloroquine Phosphate fabricated using methacrylated hyaluronic acidexperience little hydrolytic degradation over extended period likely due to the methyl groups in the methacrylates, which shield water molecules by steric hindrance. Our previous study indicated that high crosslinking density of MeHA hydrogels resulted in reduced cartilage matrix biosynthesis and more restricted cartilage matrix distribution. The hypertrophic differentiation of the hMSCs and ensuing neocartilage calcification was also enhanced in highly crosslinked MeHA hydrogels compared to hydrogels of low crosslinking density. Prior studies showed that MMP-sensitive PEG hydrogels promoted neocartilage development by the encapsulated chondrocytes and MSCs. Compared with PEG hydrogels, HA hydrogels afford a more favorable microenvironment for chondrogenesis by providing binding sites for cell surface receptors such as CD44 and CD168, activation of which are important to chondrogenesis. This study confirmed that MMP-sensitive HA hydrogels also enhanced chondrogenesis of the encapsulated hMSCs. The enhanced cell spreading and motility in the MMP-sensitive hydrogels allowed more cell-cell interactions, which are essential to chondrogenesis. The proteolytic action of MMPs also releases ECM bound growth factors that are inducer of chondrogenesis. These may have contributed to the enhanced chondrogenesis of the hMSCs in the MMP-sensitive HA hydrogels. Importantly, prior design of MMP-sensitivity in HA hydrogels was achieved by crosslinking acrylated HA with crosslinkers containing dual thiols. The resulting thiol-ether-ester bonds are potentially labile to hydrolytic degradation. A separate unpublished study revealed that the acrylated HAhydrogels quickly degraded and lost the structural integrity within a week of culture. This not only makes the acrylated HA hydrogel unsuitable for long term cell culture studies but also confounds the effect of MMP-mediated degradation on hMSCs. In contrast, maleimides react with thiols to form stable thioether bonds. A prior study demonstrated that maleimide modified HAmacromers can be crosslinked with the same thiol containing crosslinkers to form hydrogelsthat are resistant to hydrolytic degradation. In contrast to the acrylated HA hydrogels used in this study largely maintained their initial cylindrical morphology during the 4 weeks of culture without significant bulk degradation.