The potential to interfere with these processes. Early studies demonstrated that nitrogen-containing compounds, such as amino acids and ammonium ions prevent yeast cells from sporulating. Other work described the effects of chemicals that induce aneuploidy 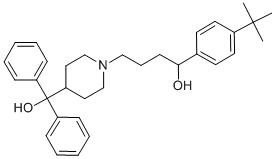 in yeast undergoing meiosis. Anti-neoplastic drugs, such as adriamycin, mitomycin C, and bleomycin were shown to disrupt the second meiotic division leading to the generation of diploid spores. These drugs, however, are not only effective during sporulation, but also abolish vegetative growth. In this study we aimed to identify chemicals that inhibit meiotic development in yeast but do not interfere with vegetative growth. We profiled a library of 446 drugs from the NIH clinical collection with two sporulation assays, and generated sensitivity profiles of growing and sporulating cells for each of these chemicals. This approach identified 12 potent, sporulation-specific inhibitors, the majority of which are cationic amphiphilic drugs. We have studied the effects of one of these drugs, tripelennamine, on various meiotic landmarks and identified genes related to autophagy as hypersensitive to the drug using chemical genomic profiling. Previous analyses of meiotic mutants in yeast have shown that cells can omit certain stages of meiotic development and still produce mature meiotic products. For example spo11D mutants, that are unable to perform meiotic recombination, are still FG-4592 capable of producing mature asci. Thus, chemical compounds that for example inhibit Spo11 would not be identified with the fluorescence-based assay described above. To overcome this limitation a second screening approach was employed. This approach is based on a hetero-allelic reporter system that has been used by others to measure meiotic reciprocal recombination, crossover and non-crossover recombination, and recombination frequencies. A strain harboring the his4 mutant allelesis unable to grow in the absence of histidine. Upon meiotic recombination between the two alleles, one of the four meiotic products will receive a functional HIS4 allele, generating a histidine-prototrophic cell that is capable of growing in the absence of histidine. This event is facilitated by the presence of two recombination hot-spots located within the HIS4 openreading frame. The production of histidine-prototrophs can be monitored by transferring aliquots of sporulating cells to media lacking histidine. Compounds that inhibit entry into meiosis, premeiotic DNA replication or recombination will suppress recombination between the his4 alleles and will therefore suppress the generation of such prototrophs. To validate this reporter assay, a proof-of-concept experiment was performed in which different concentrations of ammonium sulfate were added to his4x/his4B harboring cells upon induction of meiosis. After 5 hours of sporulation, where most cells have ABT-199 1257044-40-8 undergone pre-meiotic DNA-synthesis and meiotic recombination but have not undergone the commitment and can therefore return to growth, aliquots of the cultures were plated onto agar plates lacking histidine. As expected, the quantity of histidineprototrophic cells increased with decreasing concentrations of ammonium sulfate in the media. Results from this assay correlated with those from the fluorescence-based assay: 2, 1, and 0.5 mM of ammonium sulfate suppressed colony formation; lower concentrations of ammonium sulfate did not interfere with meiotic recombination and hence colony growth. Note, that in addition to compounds that specifically inhibit meiotic recombination and/or spore formation, the two screening assays described here will also identify compounds that are cytotoxic in cells undergoing these processes.
in yeast undergoing meiosis. Anti-neoplastic drugs, such as adriamycin, mitomycin C, and bleomycin were shown to disrupt the second meiotic division leading to the generation of diploid spores. These drugs, however, are not only effective during sporulation, but also abolish vegetative growth. In this study we aimed to identify chemicals that inhibit meiotic development in yeast but do not interfere with vegetative growth. We profiled a library of 446 drugs from the NIH clinical collection with two sporulation assays, and generated sensitivity profiles of growing and sporulating cells for each of these chemicals. This approach identified 12 potent, sporulation-specific inhibitors, the majority of which are cationic amphiphilic drugs. We have studied the effects of one of these drugs, tripelennamine, on various meiotic landmarks and identified genes related to autophagy as hypersensitive to the drug using chemical genomic profiling. Previous analyses of meiotic mutants in yeast have shown that cells can omit certain stages of meiotic development and still produce mature meiotic products. For example spo11D mutants, that are unable to perform meiotic recombination, are still FG-4592 capable of producing mature asci. Thus, chemical compounds that for example inhibit Spo11 would not be identified with the fluorescence-based assay described above. To overcome this limitation a second screening approach was employed. This approach is based on a hetero-allelic reporter system that has been used by others to measure meiotic reciprocal recombination, crossover and non-crossover recombination, and recombination frequencies. A strain harboring the his4 mutant allelesis unable to grow in the absence of histidine. Upon meiotic recombination between the two alleles, one of the four meiotic products will receive a functional HIS4 allele, generating a histidine-prototrophic cell that is capable of growing in the absence of histidine. This event is facilitated by the presence of two recombination hot-spots located within the HIS4 openreading frame. The production of histidine-prototrophs can be monitored by transferring aliquots of sporulating cells to media lacking histidine. Compounds that inhibit entry into meiosis, premeiotic DNA replication or recombination will suppress recombination between the his4 alleles and will therefore suppress the generation of such prototrophs. To validate this reporter assay, a proof-of-concept experiment was performed in which different concentrations of ammonium sulfate were added to his4x/his4B harboring cells upon induction of meiosis. After 5 hours of sporulation, where most cells have ABT-199 1257044-40-8 undergone pre-meiotic DNA-synthesis and meiotic recombination but have not undergone the commitment and can therefore return to growth, aliquots of the cultures were plated onto agar plates lacking histidine. As expected, the quantity of histidineprototrophic cells increased with decreasing concentrations of ammonium sulfate in the media. Results from this assay correlated with those from the fluorescence-based assay: 2, 1, and 0.5 mM of ammonium sulfate suppressed colony formation; lower concentrations of ammonium sulfate did not interfere with meiotic recombination and hence colony growth. Note, that in addition to compounds that specifically inhibit meiotic recombination and/or spore formation, the two screening assays described here will also identify compounds that are cytotoxic in cells undergoing these processes.