L-tryptophan supplementation was given to manipulate the long-term sequelae of early-life programming by undernutrition. We found that the effects of this Tofacitinib dietary intervention can be monitored non invasively by circadian sampling of blood Dglucose. Expressions of PERIOD1 protein by synchronized primary cell lines established from young rats were altered according to diet and L-tryptophan supplementation. However the capacity of colonization at seeding and the adhesion potentials of primary cells were clearly altered in rats born from mothers fed on dietary protein restriction, irrespectively of the supplementation with L-tryptophan. The body weight means 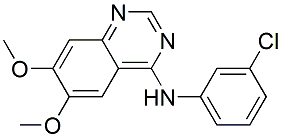 of low-protein groups in our experiment remain lower than control groups until the end of the observation period, but, the weight of visceral fat of undernourished offspring was similar to control group at the end of experiment. L-tryptophan supplementation between D-12 and D-21 did not alter the catchup growth nor the absolute food consumption of male offspring from weaning to the end of growth period. The daily food intake of rat pups measured between Day 39 and Day-42 showed that the food consumption of the LP group is lower than the CP group irrespectively of a supplementation by Ltryptophan. These observations on nursing rats with offspring are similar to results obtained in lactating sows. In addition, the relative food intake was higher for the LP group during the night cycle but the relative intakes of both groups were similar during the light cycle. These data are consistent with the temporary hyperphagy that we previously demonstrated in undernourished rat pups. A daily bolus of L-tryptophan between D-12 and D-21 did alter the level of D-glucose both in LPT and CPT groups. Our D-glucose profiles displayed a maximum BYL719 around 16 h Zeitgeber time like the profiles obtained on plasma D-glucose titration. However a significant circadian rhythm of Dglucose oscillation was only obtained with the control-fed group receiving a perinatal bolus of L-tryptophan. Results shown on Figures 3 and 5 suggest that there are a long-term effect of tryptophan supplementation on rats enduring perinatal undernutrition as well as on control-fed rats. As shown on Figure 4, we found a significant difference on the cycle of food intake during 4 consecutive days of observations on rats exposed to perinatal undernutrition and receiving a daily bolus of L-tryptophan. These results are suggesting that our supplementation triggered discrete phenotypic alterations. We think that our results indicate that milk formulas designed to improve sleep-wake cycles of babies have to be tested on rat models under several conditions of feeding to check for global phenotypic consequences. Beside oral gavage, Ltryptophan supplementation has to be tested from birth in formulated milk by using gastrostomized rat pups or with subcutaneous or intraperitonal injections. In any case, subsequently to our experiment, testing formula fortified with Ltryptophan on cerebellum gene expression of nursing rat neonates is clearly insufficient. To explore whether our supplementation with L-tryptophan interacted with the influence of perinatal undernutrition on male somatic cells, we have selected primary cells from the tip of the tail of every rat. We have tested the capacity of these primary cells to adhere and colonize plastics and the diversity of phenotypes selected from the biopsies.
of low-protein groups in our experiment remain lower than control groups until the end of the observation period, but, the weight of visceral fat of undernourished offspring was similar to control group at the end of experiment. L-tryptophan supplementation between D-12 and D-21 did not alter the catchup growth nor the absolute food consumption of male offspring from weaning to the end of growth period. The daily food intake of rat pups measured between Day 39 and Day-42 showed that the food consumption of the LP group is lower than the CP group irrespectively of a supplementation by Ltryptophan. These observations on nursing rats with offspring are similar to results obtained in lactating sows. In addition, the relative food intake was higher for the LP group during the night cycle but the relative intakes of both groups were similar during the light cycle. These data are consistent with the temporary hyperphagy that we previously demonstrated in undernourished rat pups. A daily bolus of L-tryptophan between D-12 and D-21 did alter the level of D-glucose both in LPT and CPT groups. Our D-glucose profiles displayed a maximum BYL719 around 16 h Zeitgeber time like the profiles obtained on plasma D-glucose titration. However a significant circadian rhythm of Dglucose oscillation was only obtained with the control-fed group receiving a perinatal bolus of L-tryptophan. Results shown on Figures 3 and 5 suggest that there are a long-term effect of tryptophan supplementation on rats enduring perinatal undernutrition as well as on control-fed rats. As shown on Figure 4, we found a significant difference on the cycle of food intake during 4 consecutive days of observations on rats exposed to perinatal undernutrition and receiving a daily bolus of L-tryptophan. These results are suggesting that our supplementation triggered discrete phenotypic alterations. We think that our results indicate that milk formulas designed to improve sleep-wake cycles of babies have to be tested on rat models under several conditions of feeding to check for global phenotypic consequences. Beside oral gavage, Ltryptophan supplementation has to be tested from birth in formulated milk by using gastrostomized rat pups or with subcutaneous or intraperitonal injections. In any case, subsequently to our experiment, testing formula fortified with Ltryptophan on cerebellum gene expression of nursing rat neonates is clearly insufficient. To explore whether our supplementation with L-tryptophan interacted with the influence of perinatal undernutrition on male somatic cells, we have selected primary cells from the tip of the tail of every rat. We have tested the capacity of these primary cells to adhere and colonize plastics and the diversity of phenotypes selected from the biopsies.
Category: MAPK Inhibitor Library
Effects of cocaine on each transporter and other biological ramifications of the loss of activity of monoamine transporters
In previous work we examined consequences of DAT and SERT KO on the functional connectivity of prefrontal cortex neuronal circuitry, brain morphology, and Y-27632 dihydrochloride metabolite levels. While there is some discussion about the exact definition of ‘reward circuits’, one generally accepted part of this idea includes a network linking brainstem and meso-corticallimbic structures. In this work we concentrate on the prefrontalventral striatopallidal circuit implicated in disorders involving the reward pathway and executive functions. Localized injection of manganese, an MR contrast agent well-established as a SB431542 inquirer circuitry tracer, into the prefrontal cortex followed by timelapse whole brain MR imaging enabled quantification of the functional connectivity of the circuitry. We have previously reported more robust connectivity between the PFC and posterior structures in SERT KO mice than in wildtype mice. In DAT KO mice the same procedure revealed preservation of cortico-striatalthalamic connectivity, but diminished robustness in reward circuitry distal to the thalamus. Structural and metabolic MR analysis revealed no significant differences between these knockouts and wildtype controls. Hence we concluded that lifelong transporter deficits primarily affect the degree of activity in these circuits without major large scale anatomical or metabolic disturbances. Here we extend this investigation to the NET KO mouse using the same experimental approach as for the other two monoamine transporter knockout strains, which completes our examination of the effects of gene knockout of the three major molecular targets of cocaine. Magnetic resonance spectroscopy of the striatum was used to measure the concentration of several small molecule metabolites present at millimolar or greater concentrations that have been associated with disease states. Diffusion tensor imaging was employed to investigate differences in brain structure between NET KO and WT littermate mice using tensor based morphometry to identify local structural differences between the KO and WT mice. This study reports the effects of life-long deletion of NET on metabolite levels, brain structure and neuronal connectivity in the meso-cortical-limbic reward circuitry. These findings are discussed in the context of similar work in previous studies using SERT and DAT KO mice, providing a unique framework to consider the roles of these neurotransmitters in brain function and dysfunction. In this study we investigated how mice with lifelong genetic deletion of the norepinephrine transporter differ from their wildtype littermates in aspects of metabolite levels, brain morphology and neuronal circuitry associated with reward pathways. We employed a panel of magnetic resonance methods with voxelwise analyses, as well as histological confirmation. This work extends earlier examinations of serotonin and dopamine transporter knockout mice. Interactions between the dopaminergic, serotonergic and noradrenergic systems are well known. Comparison of differences detected by MEMRI in the reward circuitry of mouse strains with deletion of each of the three monoamine transporters emphasizes the synergistic complexity of these overlapping integrated systems. We 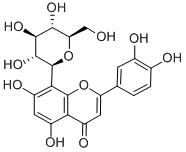 used MRS to measure metabolite levels averaged over an 8 ml volume centered in the striatum. Only N-acetyl aspartate levels differed between the NET KO and wildtype mice.
used MRS to measure metabolite levels averaged over an 8 ml volume centered in the striatum. Only N-acetyl aspartate levels differed between the NET KO and wildtype mice.
The postnatal developments of the molecular by transactivating genes involved in cellular homeostasis
HIFs are heterodimeric transcription factors which have two structurally related subunits, an Orbifloxacin oxygen sensitive HIFa subunit and a constitutively expressed HIF? or ARNT subunit. Sox2 is important for pulmonary branching morphogenesis, epithelial cell differentiation and is exclusively expressed in the proximal parts of the lung. However, in mycHIF3a expressing lungs, Sox2 is present in epithelial cells of both proximal airways and certain alveoli at postnatal day 1, suggesting that Hif3a is able to induce proximal cell fate. The basal cell marker p63 is expressed in the esophagal and tracheal epithelium, and previously we showed that ectopic Sox2 expression induced the appearance of p63 positive cells in the epithelium of the bronchioles and Folinic acid calcium salt pentahydrate enlarged distal airspaces. Therefore, we analysed  the distribution of basal cells in the mycHIF3a expressing lungs and found that p63 is abnormally expressed in the alveolar epithelial cells of mycHIF3a expressing lungs, contrasting the unique expression in the trachea. Hypoxia inducible factors are an important family of proteins involved in the regulation of the cellular response to hypoxia. Its functions are required from the earliest steps of mammalian life to the correct development of multiple organs and tissues, like the placenta, trophoblast formation, bone development, heart and vascular development. The importance of the hypoxia response was shown by the identification of human mutations in the VHL-HIF pathway in different diseases. Gene ablation studies in mice have revealed in more detail the specific and important roles of the different subunits of the Hifa/Hif? heterodimers. Inactivation of the stable subunit, Hif1?, resulted in severe embryonic defects and premature death. The disruption of the different Hifa genes identified specific roles for the individual Hifa isoforms. Hif1a knockout mice die early at gestation, have multiple developmental defects in neural tubeforrmation, vascularization, heart development, neural crest migration, whereas depending on the genetic background of the mouse strain, Hif2a knockout out mice ranging from early embryonic lethality to adulthood. Early-life stressors such as maternal undernutrition, overnutrition, hypercholesterolemia, corticosteroid therapy, uteroplacental insufficiency, or hypoxia program metabolic adaptations that initially favor survival but are ultimately detrimental to adult health. In laboratory rodents, low-protein diet during gestation and lactation has been known to reduce the life expectancy of offspring. The maternal protein restriction in the rat model of In Utero Protein Restriction is one of the most extensively explored model. The low-protein fed mothers give birth to growth-restricted offspring,, and when suckled by their mothers maintained on the same low-protein diet, they remain permanently growth-restricted, despite being weaned on a normal diet. Also, early-life undernutrition is associated with higher blood tryptophan levels, brain serotonin and impairment of the serotonergic control of feeding in female adult rats. Recently, we have shown that circadian clock of the hypothalamus is altered in young rats subsequently to perinatal undernutrition, however there is no proof that this dysregulation exists in other tissues as well. In rodents, the emergence of circadian clock outputs occur during the first 2 or 3 weeks after birth.
the distribution of basal cells in the mycHIF3a expressing lungs and found that p63 is abnormally expressed in the alveolar epithelial cells of mycHIF3a expressing lungs, contrasting the unique expression in the trachea. Hypoxia inducible factors are an important family of proteins involved in the regulation of the cellular response to hypoxia. Its functions are required from the earliest steps of mammalian life to the correct development of multiple organs and tissues, like the placenta, trophoblast formation, bone development, heart and vascular development. The importance of the hypoxia response was shown by the identification of human mutations in the VHL-HIF pathway in different diseases. Gene ablation studies in mice have revealed in more detail the specific and important roles of the different subunits of the Hifa/Hif? heterodimers. Inactivation of the stable subunit, Hif1?, resulted in severe embryonic defects and premature death. The disruption of the different Hifa genes identified specific roles for the individual Hifa isoforms. Hif1a knockout mice die early at gestation, have multiple developmental defects in neural tubeforrmation, vascularization, heart development, neural crest migration, whereas depending on the genetic background of the mouse strain, Hif2a knockout out mice ranging from early embryonic lethality to adulthood. Early-life stressors such as maternal undernutrition, overnutrition, hypercholesterolemia, corticosteroid therapy, uteroplacental insufficiency, or hypoxia program metabolic adaptations that initially favor survival but are ultimately detrimental to adult health. In laboratory rodents, low-protein diet during gestation and lactation has been known to reduce the life expectancy of offspring. The maternal protein restriction in the rat model of In Utero Protein Restriction is one of the most extensively explored model. The low-protein fed mothers give birth to growth-restricted offspring,, and when suckled by their mothers maintained on the same low-protein diet, they remain permanently growth-restricted, despite being weaned on a normal diet. Also, early-life undernutrition is associated with higher blood tryptophan levels, brain serotonin and impairment of the serotonergic control of feeding in female adult rats. Recently, we have shown that circadian clock of the hypothalamus is altered in young rats subsequently to perinatal undernutrition, however there is no proof that this dysregulation exists in other tissues as well. In rodents, the emergence of circadian clock outputs occur during the first 2 or 3 weeks after birth.
In contrast with the results that both TLR1 and TLR4 were up-regulated with normal after combination chemotherapy
Furthermore, we observed an increased nuclear S100A15 3,4,5-Trimethoxyphenylacetic acid expression in lung cancer tissues not only in stage IV NSCLC compared to stage IIIB NSCLC, but also in the patients with stable or progressive disease in comparison to those with a partial response after first line combination chemotherapy with CDDP and GEM. Additionally, a high percentage of S100A15 nuclear Tulathromycin B stained cells was the only independent factor associated with three-year overall survival. This suggests that nuclear accumulation of S100A15 may be linked to metastasis potential, treatment response, and long-term outcomes. In accordance with the results published in the Human Protein Atlas website, 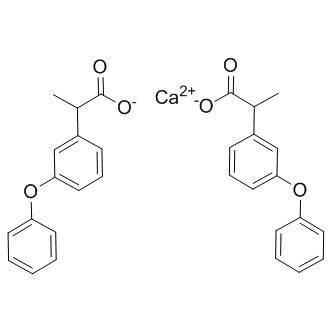 we found up-regulation of S100A15 in NSCLC cancer tissues compared to normal lung tissues surrounding the tumor. Nonetheless, S100A15 in either cancer tissues or PBMC could not distinguish AC from SCC in this small sample-size study. Additionally, quantitative RT-PCR result for S100A15 was positively correlated with cytoplasmic S100A15 staining intensity score but not with other parameters in IHC staining assessment, implying that cytoplasmic protein expression rather than nuclear protein expression may affect S100A15 expression in peripheral immune cells, such as monocyte. Disruption of the calcium signaling pathway, such as by the S100 family, has been implicated as a central mechanism in tumorigenesis, specifically tumor invasion and metastasis. Both S100A15 and S100A7 proteins have been demonstrated to be distinctly expressed in normal breast tissue and breast cancer. Since S100A15 was found to be chemotactic for both granulocytes and monocytes, and to act synergistically with highly homologous S100A7 to enhance inflammation, both proteins could influence lung tumor progression. Additionally, E. coli can modulate the human S100A15 expression of keratinocytes by recognition through TLR4, suggesting that S100A15 may play a role in innate immunity. Selective expression of S100A7 in lung SCC and large cell carcinomas has been demonstrated, but not in AC or small cell carcinomas. Nuclear accumulation of S100A7 has been reported to be associated with a poor prognosis in head and neck cancer. To the best of our knowledge, the current study is the first to demonstrate that nuclear accumulation of S100A15 may be linked to an increased risk of distant metastasis, and that S100A15 may serve as a candidate biomarker for predicting treatment response and survival. However, large scale longitudinal studies are warranted to evaluate the potential of S100A15 as a determinant of advanced tumor stage and/or a predictor of long-term outcomes in NSCLC. Stimulation with TLR7 agonists on human lung cancer cells has been shown to lead to increased tumor cell survival and chemoresistance. On the other hand, systemic administration of TLR7 agonists has also been found to induce significant antitumor activity, which could be potentiated by cyclophosphamide. In a cell culture model, TLR7 agonists were found to enhance tumor cell lysis by human gamma delta T cells. Taken together, these findings suggest that enhancing the TLR7 expression in immune cells may potentiate the antitumor effect of combination chemotherapy in advanced stage NSCLC patients.
we found up-regulation of S100A15 in NSCLC cancer tissues compared to normal lung tissues surrounding the tumor. Nonetheless, S100A15 in either cancer tissues or PBMC could not distinguish AC from SCC in this small sample-size study. Additionally, quantitative RT-PCR result for S100A15 was positively correlated with cytoplasmic S100A15 staining intensity score but not with other parameters in IHC staining assessment, implying that cytoplasmic protein expression rather than nuclear protein expression may affect S100A15 expression in peripheral immune cells, such as monocyte. Disruption of the calcium signaling pathway, such as by the S100 family, has been implicated as a central mechanism in tumorigenesis, specifically tumor invasion and metastasis. Both S100A15 and S100A7 proteins have been demonstrated to be distinctly expressed in normal breast tissue and breast cancer. Since S100A15 was found to be chemotactic for both granulocytes and monocytes, and to act synergistically with highly homologous S100A7 to enhance inflammation, both proteins could influence lung tumor progression. Additionally, E. coli can modulate the human S100A15 expression of keratinocytes by recognition through TLR4, suggesting that S100A15 may play a role in innate immunity. Selective expression of S100A7 in lung SCC and large cell carcinomas has been demonstrated, but not in AC or small cell carcinomas. Nuclear accumulation of S100A7 has been reported to be associated with a poor prognosis in head and neck cancer. To the best of our knowledge, the current study is the first to demonstrate that nuclear accumulation of S100A15 may be linked to an increased risk of distant metastasis, and that S100A15 may serve as a candidate biomarker for predicting treatment response and survival. However, large scale longitudinal studies are warranted to evaluate the potential of S100A15 as a determinant of advanced tumor stage and/or a predictor of long-term outcomes in NSCLC. Stimulation with TLR7 agonists on human lung cancer cells has been shown to lead to increased tumor cell survival and chemoresistance. On the other hand, systemic administration of TLR7 agonists has also been found to induce significant antitumor activity, which could be potentiated by cyclophosphamide. In a cell culture model, TLR7 agonists were found to enhance tumor cell lysis by human gamma delta T cells. Taken together, these findings suggest that enhancing the TLR7 expression in immune cells may potentiate the antitumor effect of combination chemotherapy in advanced stage NSCLC patients.
Putative redox enzyme that converts the hydroxymycolate products of MmaA4 into keto-mycolic acids
While these models are attractive, confirmation will require further study and more information on the structure of the proteins, their mode of interaction and how the drugs alter these interactions. Peroxisomes are single-Lomitapide Mesylate membrane bound, multifunctional and highly Tulathromycin B dynamic organelles of most eukaryotic cells, which fulfil important metabolic functions in hydrogen peroxide and lipid metabolism. Their function has also been linked to developmental processes, stress response, age-related disorders, and antiviral innate immunity. Remarkably, the peroxisomal compartment shows high plasticity and responds to developmental, environmental, and metabolic stimuli with alterations in organelle number, morphology and protein content. Peroxisomes can multiply by growth and division of pre-existing organelles or, as particularly demonstrated in yeast, can form de novo from the endoplasmic reticulum. Whereas considerable progress has been made in the identification of key factors involved in these processes, the underlying mechanisms and the regulation of these processes are only poorly understood. The assembly of peroxisomes and protein import into the organelle requires the action of essential proteins, so called peroxins, which are encoded by PEX genes. Mutations in many PEX genes have been identified as the cause of severe and often lethal peroxisome biogenesis disorders. Peroxisome formation by growth and division involves the deformation and elongation of the peroxisomal membrane, its constriction and final scission. Similar 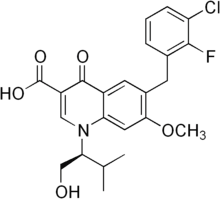 to de novo biogenesis from the ER, growth and division of peroxisomes follows a multistep maturation pathway, which results in the formation of new daughter peroxisomes. In mammals, Pex11 proteins are so far the only proteins discovered capable of deforming and elongating the peroxisomal membrane. Hence, the mechanistic details of peroxisomal growth and division and the individual functions of the human Pex11 proteins have attracted great attention as they have been linked to new disorders affecting peroxisome morphology and dynamics. It has recently been reported that Pex11 proteins feature amphipathic helices that can insert into the peroxisomal membrane, thus influencing membrane bending. In line with this, Pex11 proteins are suggested to reorganize the peroxisomal membrane prior to fission,, and to mediate interactions with the peroxisomal fission machinery. The machinery for membrane scission includes the membrane adaptor proteins Fis1 and Mff, which are involved in the recruitment of the dynamin-like large GTPase DLP1/Drp1 to constriction sites on the peroxisomal membrane. DLP1 is supposed to assemble in spiral-like structures around constricted membranes to mediate membrane scission through GTP hydrolysis leading to the formation of new peroxisomes. Interestingly, mitochondria and peroxisomes, which are metabolically linked to each other, share these key components of their division machinery supporting a closer interorganellar relationship,,, whereas Pex11 proteins are exclusively peroxisomal. Pex11 proteins are conserved amongst species; however, many organisms contain various ����isoforms���� which are poorly characterized on a functional level, and may differ in their biochemical properties. Furthermore, their membrane topology is not entirely clear and may vary amongst different species. The mammalian genome encodes for three Pex11 proteins, Pex11pa, Pex11pb, and Pex11pc, which are thought to be integral membrane proteins with their N- and C-termini facing the cytosol.
to de novo biogenesis from the ER, growth and division of peroxisomes follows a multistep maturation pathway, which results in the formation of new daughter peroxisomes. In mammals, Pex11 proteins are so far the only proteins discovered capable of deforming and elongating the peroxisomal membrane. Hence, the mechanistic details of peroxisomal growth and division and the individual functions of the human Pex11 proteins have attracted great attention as they have been linked to new disorders affecting peroxisome morphology and dynamics. It has recently been reported that Pex11 proteins feature amphipathic helices that can insert into the peroxisomal membrane, thus influencing membrane bending. In line with this, Pex11 proteins are suggested to reorganize the peroxisomal membrane prior to fission,, and to mediate interactions with the peroxisomal fission machinery. The machinery for membrane scission includes the membrane adaptor proteins Fis1 and Mff, which are involved in the recruitment of the dynamin-like large GTPase DLP1/Drp1 to constriction sites on the peroxisomal membrane. DLP1 is supposed to assemble in spiral-like structures around constricted membranes to mediate membrane scission through GTP hydrolysis leading to the formation of new peroxisomes. Interestingly, mitochondria and peroxisomes, which are metabolically linked to each other, share these key components of their division machinery supporting a closer interorganellar relationship,,, whereas Pex11 proteins are exclusively peroxisomal. Pex11 proteins are conserved amongst species; however, many organisms contain various ����isoforms���� which are poorly characterized on a functional level, and may differ in their biochemical properties. Furthermore, their membrane topology is not entirely clear and may vary amongst different species. The mammalian genome encodes for three Pex11 proteins, Pex11pa, Pex11pb, and Pex11pc, which are thought to be integral membrane proteins with their N- and C-termini facing the cytosol.