Hence, this reaction represents a physiological mechanism of the cell and not a primary pathological event. However, in contrast to hibernation, the hypometabolic state is not terminated after a definite time but rather persists and progresses. The phosphorylation of tau protein endures and in the course the actually reversible physiological reaction turns into a pathological event promoted by the large period of time of AD pathogenesis. In the neocortex of hibernating black bears we found a conformational change of tau protein. This particular interspecies difference might be the result of the considerably elevated body Lomitapide Mesylate temperature in torpor of bears. In vitro studies could demonstrate that the aggregation of tau is inhibited at low temperatures. Since an altered conformation of tau is suggested to impact the propensity of aggregation the change of protein conformation in black bears might reflect a transitional state of a physiological process and thereby highlights the limitation of that particular cellular reaction pattern. A progression may potentially yield in aggregation and tangle formation. This hypothesis is supported by the report of neurofibrillary tangle formation in aged bears. A PHF-like phosphorylation and altered isoform expression of tau protein occurs during torpor in three species of hibernators that differ greatly in body size and in the minimum brain temperatures and metabolic rates they achieve. This phosphorylation is fully reversed when animals return to normal levels of temperature and metabolism whether it is regularly during arousal intervals in small hibernators or seasonally in large hibernators. These findings indicate that a reduced binding capacity of tau may be a precondition to endure the hypometabolic state and reduced tissue temperatures during hibernation. Moreover, the interspecies 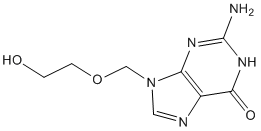 homogeneity of this reaction pattern suggests that this regulation is subject to a basal physiological mechanism of mammals. Increased neuronal tau phosphorylation in early stages of AD, therefore, may be potentially considered as physiological reaction to a reduced brain metabolic rate. However, as the result of the slow and progressive pathological process in AD, hyperphosphorylated tau protein aggregates to neurofibrillary tangles most likely leading to the degeneration of affected neurons. PHF-like tau phosphorylation in hibernation is paralleled by an aberrant synaptic plasticity and a loss of memory. Due to the cooccurrence of these elementary attributes of AD hibernating mammals represent a unique and useful model organism to study the relevance and coherencies of particular characteristics of AD pathology. This is of utmost importance for the development of potential strategies for a medical treatment and therapy of this disease. Our ability to predict the impacts of climate change on coral reef is dependent on deciphering the cellular and molecular mechanisms that drive sensitivity of corals to thermal stress and bleaching. In this respect, significant progress has been made during the past two decades towards understanding the mechanisms of bleaching. A wide array of cellular mechanisms has been proposed to explain the loss of symbionts from the coral tissue including exocytosis, pinching off, host cell detachment, and cell death pathway activation. The susceptibility to bleaching can vary greatly amongst species and geographical locations, and may be attributed to both differences in thermal tolerance among symbionts clades and sub-clades and/or the coral host and its capacity to Chloroquine Phosphate tolerate stress by employing a wide variety of protective responses. One of the first sites damaged in response to thermal stress is the symbiont chloroplast, where photosynthetic dysfunction results in the overproduction of reactive oxygen species, leading to symbiont and host cell damage, through a variety of possible mechanisms. One suite of the molecular mechanisms that results in the removal of highly compromised symbionts from stressed host tissues involves regulation of cell death pathways which play active roles in maintaining tissue homeos.
homogeneity of this reaction pattern suggests that this regulation is subject to a basal physiological mechanism of mammals. Increased neuronal tau phosphorylation in early stages of AD, therefore, may be potentially considered as physiological reaction to a reduced brain metabolic rate. However, as the result of the slow and progressive pathological process in AD, hyperphosphorylated tau protein aggregates to neurofibrillary tangles most likely leading to the degeneration of affected neurons. PHF-like tau phosphorylation in hibernation is paralleled by an aberrant synaptic plasticity and a loss of memory. Due to the cooccurrence of these elementary attributes of AD hibernating mammals represent a unique and useful model organism to study the relevance and coherencies of particular characteristics of AD pathology. This is of utmost importance for the development of potential strategies for a medical treatment and therapy of this disease. Our ability to predict the impacts of climate change on coral reef is dependent on deciphering the cellular and molecular mechanisms that drive sensitivity of corals to thermal stress and bleaching. In this respect, significant progress has been made during the past two decades towards understanding the mechanisms of bleaching. A wide array of cellular mechanisms has been proposed to explain the loss of symbionts from the coral tissue including exocytosis, pinching off, host cell detachment, and cell death pathway activation. The susceptibility to bleaching can vary greatly amongst species and geographical locations, and may be attributed to both differences in thermal tolerance among symbionts clades and sub-clades and/or the coral host and its capacity to Chloroquine Phosphate tolerate stress by employing a wide variety of protective responses. One of the first sites damaged in response to thermal stress is the symbiont chloroplast, where photosynthetic dysfunction results in the overproduction of reactive oxygen species, leading to symbiont and host cell damage, through a variety of possible mechanisms. One suite of the molecular mechanisms that results in the removal of highly compromised symbionts from stressed host tissues involves regulation of cell death pathways which play active roles in maintaining tissue homeos.
Category: MAPK Inhibitor Library
Apoptosis is one of the main types of programmed cell death and is active in response to various physiological and pathological situations
In the basal phylum Cnidaria, the initiation of cell death has been shown to occur during development, hyperthermic stress, ultraviolet radiation, chemical induction immune response to disease and onset of symbiosis. The apoptotic pathway is dependent upon the activation of proteolytic enzymes named caspases. Caspase activation can be initiated through either the extrinsic pathway or the intrinsic pathway which involves the permeabilisation of the mitochondrial outer membrane and subsequent release of 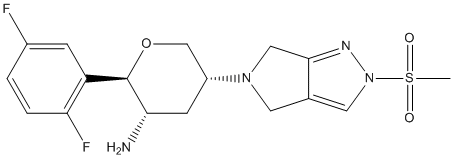 cytochrome c into the cytosol. The regulation of the mitochondria intrinsic pathway is Ginsenoside-F2 performed by a Tulathromycin B complex protein network in which the B-cell lymphoma protein-2 family members form a central checkpoint that determines whether a cell lives or dies. Bcl-2 proteins are divided between pro-apoptotic and antiapoptotic members according to their Bcl-2 Homology domains and associated function. Among the pro-apoptotic members, Bcl-2-associated X and Bcl-2antagonist/killer-1 promote apoptosis through their oligomerization and insertion in the mitochondrial outer membrane where they form large pores. Bax and Bak activation is controlled by the interplay and heterodimer formation with antiapoptotic members, such as Bcl-2. Bcl-2 is potentially the most characterised anti-apoptotic member, and functions by regulating transcription, caspase activation, mitochondrial membrane pore formation, intracellular Ca2+ homeostasis and by increasing cellular resistance to oxidative stress. Consequently, it has been suggested that the ratios between antiapoptotic Bcl-2 and pro-apoptotic Bax and Bak may be more important than either promoter alone in determining apoptosis. These ratios can therefore be used as prognostic markers to study apoptosis regulation. Although direct evidence of mitochondrial outer membrane permeabilisation is still lacking in the more basal phyla, extensive studies have revealed that the general outline of the apoptotic machinery is conserved among metazoans. In the Cnidaria, a basal metazoan phylum, both anti-apoptotic and pro-apoptotic-like Bcl-2 sequences have been identified in the hydrozoan Hydra magnipapillata, the non-symbiotic anthozoan, Nematostella vectensis, the symbiotic anthozoans Aiptasia pulchella and the reef building corals Acropora aspera and A. millepora. Furthermore, there is now increasing evidence that activation of caspase-dependent apoptosis plays a role during cnidarian bleaching. However, a direct link between the molecular regulation of the Bcl-2 family of apoptotic mediators and the activation of cell death in corals is still yet to be shown. The present study aimed to provide an integrated picture of the regulation of the mitochondrial apoptotic cascade by Bcl-2 family members within the reef building coral A. millepora. In this respect, we used quantitative Real-Time PCR to monitor the potential initiation of apoptosis by differential expression of Bcl-2, Bax and Bak, fluorometric assay of downstream caspase 3-like activity to measure the execution of apoptotic events and TUNEL labelling to detect the completion of cell death via DNA fragmentation. Regulation of these apoptotic mediators was investigated in complement to indicators of coral bleaching in response to defined stressors and different thermal treatments. This work aims to bring further insight into the characterization of the apoptotic pathways and their functional activation in symbiotic Cnidaria during thermal stress. Deciphering the cellular and molecular mechanisms that determine the sensitivity of corals to thermal stress represents an important step to predicting and understanding the impacts of climate change on coral reefs. In this respect, apoptosis is an important and fundamental component of the response of corals to thermal stress. Indeed, several studies focusing either on functional activation of apoptosis or transcriptomics in Cnidaria have separately demonstrated the involvement of apoptosis in response to rising seawater temperature.
cytochrome c into the cytosol. The regulation of the mitochondria intrinsic pathway is Ginsenoside-F2 performed by a Tulathromycin B complex protein network in which the B-cell lymphoma protein-2 family members form a central checkpoint that determines whether a cell lives or dies. Bcl-2 proteins are divided between pro-apoptotic and antiapoptotic members according to their Bcl-2 Homology domains and associated function. Among the pro-apoptotic members, Bcl-2-associated X and Bcl-2antagonist/killer-1 promote apoptosis through their oligomerization and insertion in the mitochondrial outer membrane where they form large pores. Bax and Bak activation is controlled by the interplay and heterodimer formation with antiapoptotic members, such as Bcl-2. Bcl-2 is potentially the most characterised anti-apoptotic member, and functions by regulating transcription, caspase activation, mitochondrial membrane pore formation, intracellular Ca2+ homeostasis and by increasing cellular resistance to oxidative stress. Consequently, it has been suggested that the ratios between antiapoptotic Bcl-2 and pro-apoptotic Bax and Bak may be more important than either promoter alone in determining apoptosis. These ratios can therefore be used as prognostic markers to study apoptosis regulation. Although direct evidence of mitochondrial outer membrane permeabilisation is still lacking in the more basal phyla, extensive studies have revealed that the general outline of the apoptotic machinery is conserved among metazoans. In the Cnidaria, a basal metazoan phylum, both anti-apoptotic and pro-apoptotic-like Bcl-2 sequences have been identified in the hydrozoan Hydra magnipapillata, the non-symbiotic anthozoan, Nematostella vectensis, the symbiotic anthozoans Aiptasia pulchella and the reef building corals Acropora aspera and A. millepora. Furthermore, there is now increasing evidence that activation of caspase-dependent apoptosis plays a role during cnidarian bleaching. However, a direct link between the molecular regulation of the Bcl-2 family of apoptotic mediators and the activation of cell death in corals is still yet to be shown. The present study aimed to provide an integrated picture of the regulation of the mitochondrial apoptotic cascade by Bcl-2 family members within the reef building coral A. millepora. In this respect, we used quantitative Real-Time PCR to monitor the potential initiation of apoptosis by differential expression of Bcl-2, Bax and Bak, fluorometric assay of downstream caspase 3-like activity to measure the execution of apoptotic events and TUNEL labelling to detect the completion of cell death via DNA fragmentation. Regulation of these apoptotic mediators was investigated in complement to indicators of coral bleaching in response to defined stressors and different thermal treatments. This work aims to bring further insight into the characterization of the apoptotic pathways and their functional activation in symbiotic Cnidaria during thermal stress. Deciphering the cellular and molecular mechanisms that determine the sensitivity of corals to thermal stress represents an important step to predicting and understanding the impacts of climate change on coral reefs. In this respect, apoptosis is an important and fundamental component of the response of corals to thermal stress. Indeed, several studies focusing either on functional activation of apoptosis or transcriptomics in Cnidaria have separately demonstrated the involvement of apoptosis in response to rising seawater temperature.
ATL protein from tobacco induced during the defense response mediated by the interaction between Avr9 and Cf9
Studies characterizing the roles of several ubiquitin E3  ligases in defense have begun to provide clues about the regulation of pathogen-induced signaling. For instance the rice resistance protein Xa21 has been shown to interact with an E3 ubiquitin ligase XB3. Interaction Gomisin-D between XB3 and Xa21 is required for the accumulation of the XA21 protein and is necessary for Xa21-mediated resistance to Xanthomonas oryzae pv. oryza in rice. A RING-finger type protein from pepper CaRFP1 was shown to physically interact with PR-1 protein in leaves of plants after infection with both bacterial and fungal pathogens. Over-expression of CaRFP1 in transgenic Arabidopsis conferred disease susceptibility to Pseudomonas syringae pv. tomato and reduced PR-2 and PR-5 expression suggesting that CaRFP1 is an E3 ligase that targets PR proteins. E3 ligases also appear to play a prominent role in elicitormediated defense responses. In particular, members of the ATL gene family have been shown to be activated by elicitors and to play important roles in defense pathways. The Arabidopsis ATL gene family contains 80 members and is a conserved group of RING zinc-finger proteins that encode putative E3 ubiquitin ligases. ATL2 and ATL6 in Arabidopsis and EL5 in rice, all encoding RING-finger type E3 ligases, have been shown to be rapidly induced in response to the elicitor chitin. Recent work by Hondo et al. demonstrated that the tomato ortholog of Arabidopsis ATL2, LeATL6, responded to cell wall protein fraction elicitor from the biocontrol agent Pythium oligandrum and appeared to regulate the jasmonic aciddependent defense gene expression. In a screen for chitinresponsive genes in Arabidopsis, we identified an ATL family member, ATL9, that responded strongly to chitin treatment. Loss-of-function mutations in this gene resulted in increased susceptibility to the powdery mildew pathogen, Golovinomyces cichoracearum. Our results here confirm that ATL9 is an E3 ubiquitin ligase and show that it is localized to the endoplasmic reticulum. ATL9 expression is induced by infection with G. cichoracearum and ATL9 function is required for basal defense against this biotrophic pathogen. Interestingly, ATL9 expression appears to be dependent on NADPH oxidases and mutations in ATL9 lead to an impairment in the ability of plants to produce reactive oxygen species after infection. Expression profiling of atl9 revealed a complex interplay between chitin-mediated signaling and other defense pathways. Recent work has highlighted the ubiquitin-proteasome system and its associated E3 ubiquitin ligases as regulators of the plant defense response and it is clear that these proteins play an important part in disease resistance. In the current study we have shown that ATL9 is a 4-(Benzyloxy)phenol RING-type E3 ubiquitin ligase strongly induced by chitin. The ATL gene family encodes a group of proteins that share three specific characteristics: 1) rapid induction after elicitor treatment, 2) a highly conserved RING-H2 zinc-finger domain with at least six cysteines and three histidines conserved and 3) at least one amino-terminal transmembrane domain. Most members of the ATL gene family are predicted to function as single subunit E3 ubiquitin ligases with seventeen members of the ATL family known to be expressed in Arabidopsis. Although determination of a common function for them is still in progress, mutations in members of the ATL family have been shown to have an altered defense response to pathogens. The ATL2 gene was shown to be specifically induced by chitin but not by other elicitors of classic defense pathways. Constitutive over-expression mutants of ATL2 induced high levels of pathogen-related genes such as NPR1-1 and the phenylpropanoid biosynthetic enzymes phenylalanine ammonia lyase and chalcone synthase. The EL5 gene in rice is also a member of the ATL family and is rapidly and transiently induced by chitin. EL5, like ATL9, is an E3 ubiquitin ligase and is hypothesized to play a role in defense responses through protein turnover via the UPS.
ligases in defense have begun to provide clues about the regulation of pathogen-induced signaling. For instance the rice resistance protein Xa21 has been shown to interact with an E3 ubiquitin ligase XB3. Interaction Gomisin-D between XB3 and Xa21 is required for the accumulation of the XA21 protein and is necessary for Xa21-mediated resistance to Xanthomonas oryzae pv. oryza in rice. A RING-finger type protein from pepper CaRFP1 was shown to physically interact with PR-1 protein in leaves of plants after infection with both bacterial and fungal pathogens. Over-expression of CaRFP1 in transgenic Arabidopsis conferred disease susceptibility to Pseudomonas syringae pv. tomato and reduced PR-2 and PR-5 expression suggesting that CaRFP1 is an E3 ligase that targets PR proteins. E3 ligases also appear to play a prominent role in elicitormediated defense responses. In particular, members of the ATL gene family have been shown to be activated by elicitors and to play important roles in defense pathways. The Arabidopsis ATL gene family contains 80 members and is a conserved group of RING zinc-finger proteins that encode putative E3 ubiquitin ligases. ATL2 and ATL6 in Arabidopsis and EL5 in rice, all encoding RING-finger type E3 ligases, have been shown to be rapidly induced in response to the elicitor chitin. Recent work by Hondo et al. demonstrated that the tomato ortholog of Arabidopsis ATL2, LeATL6, responded to cell wall protein fraction elicitor from the biocontrol agent Pythium oligandrum and appeared to regulate the jasmonic aciddependent defense gene expression. In a screen for chitinresponsive genes in Arabidopsis, we identified an ATL family member, ATL9, that responded strongly to chitin treatment. Loss-of-function mutations in this gene resulted in increased susceptibility to the powdery mildew pathogen, Golovinomyces cichoracearum. Our results here confirm that ATL9 is an E3 ubiquitin ligase and show that it is localized to the endoplasmic reticulum. ATL9 expression is induced by infection with G. cichoracearum and ATL9 function is required for basal defense against this biotrophic pathogen. Interestingly, ATL9 expression appears to be dependent on NADPH oxidases and mutations in ATL9 lead to an impairment in the ability of plants to produce reactive oxygen species after infection. Expression profiling of atl9 revealed a complex interplay between chitin-mediated signaling and other defense pathways. Recent work has highlighted the ubiquitin-proteasome system and its associated E3 ubiquitin ligases as regulators of the plant defense response and it is clear that these proteins play an important part in disease resistance. In the current study we have shown that ATL9 is a 4-(Benzyloxy)phenol RING-type E3 ubiquitin ligase strongly induced by chitin. The ATL gene family encodes a group of proteins that share three specific characteristics: 1) rapid induction after elicitor treatment, 2) a highly conserved RING-H2 zinc-finger domain with at least six cysteines and three histidines conserved and 3) at least one amino-terminal transmembrane domain. Most members of the ATL gene family are predicted to function as single subunit E3 ubiquitin ligases with seventeen members of the ATL family known to be expressed in Arabidopsis. Although determination of a common function for them is still in progress, mutations in members of the ATL family have been shown to have an altered defense response to pathogens. The ATL2 gene was shown to be specifically induced by chitin but not by other elicitors of classic defense pathways. Constitutive over-expression mutants of ATL2 induced high levels of pathogen-related genes such as NPR1-1 and the phenylpropanoid biosynthetic enzymes phenylalanine ammonia lyase and chalcone synthase. The EL5 gene in rice is also a member of the ATL family and is rapidly and transiently induced by chitin. EL5, like ATL9, is an E3 ubiquitin ligase and is hypothesized to play a role in defense responses through protein turnover via the UPS.
The secretory pathway by noroviruses direct antagonism of the secretory pathway
Both of these possibilities are currently under investigation as potential targets for p22 and as scaffolding factors upon which noroviruses may anchor their genome replication. The data presented in this study support, but do not prove, that p22 targets COPII vesicle trafficking; this is further complicated by the examination of cells by immunofluorescence primarily at 24 hpt, which may not reflect steady-state localization. Proving this mechanism will require demonstrating a specific binding to or inhibition of the trafficking of COPII vesicles using in vitro assays. Unfortunately, these studies have not been possible due to the inability of producing purified p22 for such studies as well as the lack of an antibody to p22 that allows for immunoprecipitation assays. Therefore, until a direct interaction is demonstrated, it remains possible that p22 could target a non-COPII aspect of the secretory pathway to mediate inhibition. p22 does not activate Arf1 or Sar1, making targeting of the formation of COPI vesicles in the same manner as PV and CVB3 3A, or COPII vesicles by a unique approach, unlikely. A trans Golgi mechanism of action seems similarly unlikely based on lack of localization of wildtype p22 with the trans Golgi marker Gomisin-D protein golgin 97; the same is also true for possible targeting of endosomes by p22. Thus, although there are many possible alternative explanations for the observed effects of p22, specific targeting and mislocalization of COPII vesicles is at present the most likely explanation. Although no other cellular or microbial protein to date has been described to use the arrangement of a MERES motif that p22 employs to inhibit the secretory pathway, several  previously characterized secretory pathway antagonists have potential ER export signals or mimics thereof. The Eschericia coli protein NleA inhibits COPII-dependent export from the ER by direct interaction with Sec24, and the cellular proteins STAM-1 and -2, which are involved in the signaling of growth factors and cytokines, regulate Golgi architecture by interaction the Sec13/31 COPII cage components. Examination of the primary amino acid sequence of NleA and STAM-1/2 revealed motifs similar to a di-acidic ER export signal. If further studies determine these motifs directly contribute to either the cellular localization or, for STAM-1/2, proper ER export, this would provide support for the idea that ER export signals or their mimics can be used not only to facilitate ER export, but also to promote interaction with COPII vesicles to mediate specific antagonism of the secretory pathway. Both similarities and differences were noted between p22 and the picornavirus 3A protein. Both proteins localize to membranes via an amphipathic alpha helix, and both inhibit ER-to-Golgi trafficking to decrease cellular protein secretion. However, the mechanism of this shutoff appears to be quite distinct between Norwalk virus and picornaviruses. Inhibition of protein Albaspidin-AA secretion and Golgi disassembly are in some cases separable and distinct, as is the case for PV infection, whereas in other cases one will follow the other, for example during cell division. There were also clear ultrastructural similarities between cells expressing p22 and 3A in inducing the accumulation of free membranes, double-membrane vesicles and vacuoles, although cells expressing p22 did not exhibit the swelling of the ER reported for PV 3A or the crystalloid ER patterns seen after expression of the hepatitis A virus 2C and 2BC proteins that also induce significant membrane rearrangements. This further supports the similarity of p22 and 3A in secretory pathway antagonism, but through different arms of this pathway. NV p22 therefore may be more similar in the cellular effects of the hepatitis C virus NS4A/B protein, which, though less studied than PV 3A, antagonizes ERto-Golgi trafficking, and induces the accumulation of ”membranous webs,” vacuoles and double-membrane vesicles, but not ER swelling. Although all the effects of NV infection on the secretory pathway have not yet been explored, the results presented here demonstrate.
previously characterized secretory pathway antagonists have potential ER export signals or mimics thereof. The Eschericia coli protein NleA inhibits COPII-dependent export from the ER by direct interaction with Sec24, and the cellular proteins STAM-1 and -2, which are involved in the signaling of growth factors and cytokines, regulate Golgi architecture by interaction the Sec13/31 COPII cage components. Examination of the primary amino acid sequence of NleA and STAM-1/2 revealed motifs similar to a di-acidic ER export signal. If further studies determine these motifs directly contribute to either the cellular localization or, for STAM-1/2, proper ER export, this would provide support for the idea that ER export signals or their mimics can be used not only to facilitate ER export, but also to promote interaction with COPII vesicles to mediate specific antagonism of the secretory pathway. Both similarities and differences were noted between p22 and the picornavirus 3A protein. Both proteins localize to membranes via an amphipathic alpha helix, and both inhibit ER-to-Golgi trafficking to decrease cellular protein secretion. However, the mechanism of this shutoff appears to be quite distinct between Norwalk virus and picornaviruses. Inhibition of protein Albaspidin-AA secretion and Golgi disassembly are in some cases separable and distinct, as is the case for PV infection, whereas in other cases one will follow the other, for example during cell division. There were also clear ultrastructural similarities between cells expressing p22 and 3A in inducing the accumulation of free membranes, double-membrane vesicles and vacuoles, although cells expressing p22 did not exhibit the swelling of the ER reported for PV 3A or the crystalloid ER patterns seen after expression of the hepatitis A virus 2C and 2BC proteins that also induce significant membrane rearrangements. This further supports the similarity of p22 and 3A in secretory pathway antagonism, but through different arms of this pathway. NV p22 therefore may be more similar in the cellular effects of the hepatitis C virus NS4A/B protein, which, though less studied than PV 3A, antagonizes ERto-Golgi trafficking, and induces the accumulation of ”membranous webs,” vacuoles and double-membrane vesicles, but not ER swelling. Although all the effects of NV infection on the secretory pathway have not yet been explored, the results presented here demonstrate.
We demonstrate that truncation of the final residues of its vimentin binding are correlated
In summary, SERT is one of the proteins that link vimentin to the plasma membrane. Our co-IP studies in endogenous and heterologous expression systems, and IF analyses demonstrate that in 5HT stimulated platelets the level of SERT precipitated on vimentin-Ab is higher than that in control platelets, which were altered by the extracellular level of 5HT. The plasma Pimozide membrane level of SERT is altered by the rate of the  translocation transporter protein to/from the plasma membrane which is controlled through its interaction with other proteins in these pathways. It was well documented that the plasma level of 5HT plays a role in the density of SERT on the plasma membrane via PKC-mediated phosphorylation of SERT. Additionally, the C-terminus region of SERT is vital to the ability of these transporters to function. Our studies here identify a novel pathway by correlating how plasma 5HT plays a role on the translocation of SERT from the plasma membrane via using the C-terminus region of transporter. The density of SERT on the plasma membrane is modulated by its interaction with other proteins such as an adaptor protein, Hic5 plays a role in the internalization of SERT in platelets. Also, the C-terminal region of SERT was identified as a domain of interaction with the actin cytoskeleton. Relevant findings include that the C-terminal of SERT interacts with MacMARCKS, a substrate of PKC that binds to the actin cytoskeleton, and the fact that PKC modulators, such as bPMA, modulate the activity of SERT. Our data further support this contention by demonstrating that C-terminal truncated forms of SERT show a loss of functional membrane trafficking. This loss of function may relate to the level of interact between SERT and cytoskeleton network. Our studies with SERT in transient transfection systems reveal that the truncation of various lengths of the C-terminus altered the 5HT uptake rate of SERT transporters. Truncation of the final 26 and 20 amino acid residues of SERT completely abolished uptake, whereas truncation of the final 14 and 6 residues resulted in a 12% to 18% loss in transport capacity as compared to full Gentamycin Sulfate length SERT. These results agree with published reports for NET and SERT, which demonstrate that truncation of the Cterminus abolished the uptake rates of these transporters. However, a single residue removal from the C-terminus of NET caused a 60% reduction in uptake capacity.
translocation transporter protein to/from the plasma membrane which is controlled through its interaction with other proteins in these pathways. It was well documented that the plasma level of 5HT plays a role in the density of SERT on the plasma membrane via PKC-mediated phosphorylation of SERT. Additionally, the C-terminus region of SERT is vital to the ability of these transporters to function. Our studies here identify a novel pathway by correlating how plasma 5HT plays a role on the translocation of SERT from the plasma membrane via using the C-terminus region of transporter. The density of SERT on the plasma membrane is modulated by its interaction with other proteins such as an adaptor protein, Hic5 plays a role in the internalization of SERT in platelets. Also, the C-terminal region of SERT was identified as a domain of interaction with the actin cytoskeleton. Relevant findings include that the C-terminal of SERT interacts with MacMARCKS, a substrate of PKC that binds to the actin cytoskeleton, and the fact that PKC modulators, such as bPMA, modulate the activity of SERT. Our data further support this contention by demonstrating that C-terminal truncated forms of SERT show a loss of functional membrane trafficking. This loss of function may relate to the level of interact between SERT and cytoskeleton network. Our studies with SERT in transient transfection systems reveal that the truncation of various lengths of the C-terminus altered the 5HT uptake rate of SERT transporters. Truncation of the final 26 and 20 amino acid residues of SERT completely abolished uptake, whereas truncation of the final 14 and 6 residues resulted in a 12% to 18% loss in transport capacity as compared to full Gentamycin Sulfate length SERT. These results agree with published reports for NET and SERT, which demonstrate that truncation of the Cterminus abolished the uptake rates of these transporters. However, a single residue removal from the C-terminus of NET caused a 60% reduction in uptake capacity.