Therefore, reduced plasma apelin levels may be associated with reductions of NO bioavailability in older adults, and this may in turn result in increased arterial stiffness. Additionally, in patients with type 2 diabetes mellitus, regular exercise training elevates circulating apelin levels, and higher levels of physical activity caused larger increases in apelin levels than lower levels of activity. Furthermore, a study in hypertensive rats showed that exercise training promotes mRNA expression and tissue concentration of apelin in the aorta. However, the association between Danshensu aerobic exercise traininginduced changes in arterial stiffness and circulating apelin level in healthy middle-aged and older Ganoderic-acid-G adults remains unclear. We hypothesized that aerobic exercise training would elevate plasma apelin levels along with plasma NOx levels in middle-aged and older adults, and that this increase in apelin may be participated in endurance exercise training-induced reduction of arterial stiffness. To test our hypothesis, we measured plasma apelin levels, nitrite/nitrate concentrations, and arterial stiffness in middle-aged and older adults using a randomized controlled exercise intervention trial. This study investigated the effects of regular aerobic exercise on apelin concentrations in middle-aged and older adults before and after 8-week aerobic exercise training. After the exercise training intervention, arterial stiffness decreased, concomitantly, plasma apelin levels elevated along with plasma NOx levels. By contrast, there were no significant changes in these parameters in the sedentary control group. Furthermore, the effect of training on carotid b-stiffness was negatively correlated with the effect on plasma apelin levels. Thus, an elevation of plasma apelin levels is associated with a concomitant decrease in arterial stiffness through aerobic exercise training in middle-aged and older adults. Additionally, the effect of training on plasma NOx levels was positively correlated with the effect on plasma apelin levels. 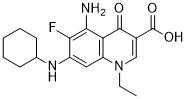 Several studies have suggested that increases in NO production resulting from endurance exercise training may be a causal factor in reduction of arterial stiffness risks, in both humans and animals. Therefore, these results suggest that arterial NO bioavailability via apelin may be participated in variation of arterial stiffness in middle-aged and older adults. In a previous study, circulating apelin levels were increased by 6 months of aerobic training consisting of walking, treadmill running, cycling, or calisthenics at 60�C70% of maximal heart rate for 60 min, 4 days/week, whereas 8-types of resistance training at 60�C80% of one repetition maximum, 4 days/week, did not change apelin levels in patients with type 2 diabetes mellitus. Patients with higher physical activity had higher plasma apelin levels than less physically physical active patients. In animal studies using hypertensive model rats, 9-week swimming training normalized mRNA expression and tissue concentration of apelin in aorta, along with apelin plasma levels. Previously, however, the effect of regular aerobic exercise on apelin concentration in middle-aged and older adults had remained unclear.
Several studies have suggested that increases in NO production resulting from endurance exercise training may be a causal factor in reduction of arterial stiffness risks, in both humans and animals. Therefore, these results suggest that arterial NO bioavailability via apelin may be participated in variation of arterial stiffness in middle-aged and older adults. In a previous study, circulating apelin levels were increased by 6 months of aerobic training consisting of walking, treadmill running, cycling, or calisthenics at 60�C70% of maximal heart rate for 60 min, 4 days/week, whereas 8-types of resistance training at 60�C80% of one repetition maximum, 4 days/week, did not change apelin levels in patients with type 2 diabetes mellitus. Patients with higher physical activity had higher plasma apelin levels than less physically physical active patients. In animal studies using hypertensive model rats, 9-week swimming training normalized mRNA expression and tissue concentration of apelin in aorta, along with apelin plasma levels. Previously, however, the effect of regular aerobic exercise on apelin concentration in middle-aged and older adults had remained unclear.
Category: MAPK Inhibitor Library
This effect was blocked in the presence of a NOS inhibitor suggesting that apelin causes vasodilation
Another microRNA related to cell differentiation and proliferation is miR145. La Rocca et al. demonstrated that miR-145 expression is increased during cell differentiation. A 2009 study conducted on cell cultures of pre-adipocytes and differentiating adipocytes of obese individuals demonstrated that the expression of miR-145 was greatly reduced during cell differentiation. In the present study, the expression of miR-143 and miR-145 did not differ between obese individuals and non-obese controls. We expected to detect an increased expression of miR-143 and miR-145 in all tissues of the obese group compared to control since these microRNAs are related to increased cell differentiation and proliferation, but this was not the case. On the other hand, there was a negative correlation between the expression of miR-145 and LEPR in the omentum of obese patients. In a critical evaluation of this article, the limited number of patients included in each group, mainly because of the limited grant dedicated to it, must have interfered in the statistical analysis. New study with more patients is now being planned and also with the possibility to include other microRNAs. On the basis of the present data, we may conclude that there was a negative correlation between the expression of miR-145 and LEPR in the omentum of obese patients. This allows us to Saikosaponin-C affirm that miR-145 may play a role in the regulation of obesity. New studies, with larger casuistic, are needed to confirm the real envolvement of the microRNAs with obesity. Alterations in arterial structure and function occur with advancing age in healthy individuals, and the aging-induced decrease in endothelial function contributes to the increases in arterial stiffness. Several studies have shown that arterial stiffness is lower in physically active individuals compared with sedentary individuals. Furthermore, aerobic exercise training reduces arterial stiffness, which increases with advancing age. Thus, regular aerobic exercise prevents or reduces arterial stiffness. Nitric oxide, which is produced from Larginine by endothelial NO synthase in endothelial cells, contributes to the underlying mechanism of this effect of exercise. NO Rosiridin causes vasodilation and inhibits the development of arteriosclerosis and atherosclerosis. Aging impairs arterial eNOS protein and mRNA expression, however, eNOS expression levels are increased by endurance exercise training in aged rats. Moreover, in middle-aged and older woman, moderate regular exercise training elevates plasma NOx levels with reduction of blood pressure. Thus, aging impairs NO 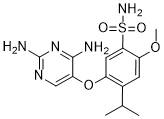 bioavailability, and it may result in increased arterial stiffness. Recently, apelin has been detected in several tissues, such as white adipose tissue, kidney, heart, and vessel. Apelin is initially synthesized as preproapelin, which consists of 77 aminoacid residues. Following enzymatic cleavage, the C-terminus is released into the circulation as the biologically active fragment, apelin. Apelin acts via APJ receptor in the expressing endothelial cells. Clinical evidence suggests that plasma apelin levels are generally lower in patients with cardiovascular diseases, such as heart failure and hypertension. In animal studies, apelin administration decreased blood pressure in normal and hypertensive rats.
bioavailability, and it may result in increased arterial stiffness. Recently, apelin has been detected in several tissues, such as white adipose tissue, kidney, heart, and vessel. Apelin is initially synthesized as preproapelin, which consists of 77 aminoacid residues. Following enzymatic cleavage, the C-terminus is released into the circulation as the biologically active fragment, apelin. Apelin acts via APJ receptor in the expressing endothelial cells. Clinical evidence suggests that plasma apelin levels are generally lower in patients with cardiovascular diseases, such as heart failure and hypertension. In animal studies, apelin administration decreased blood pressure in normal and hypertensive rats.
ERC1 was predominantly localized to the cytoplasm in mock infected cells
By 24 hours p.i. the amount of ERC1 was 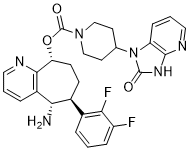 observably diminished in DENV-2 infected cells and by 48 hours p.i. was barely detectable. PRAF2 accumulated most strongly in the cytoplasm of the mock infected cells, showing a punctate distribution, supporting the previous observation that the protein was localized to the ER and transGolgi network. By contrast, in DENV-2 infected cells, PRAF2 appeared to co-localize with the viral E protein at 24 hours p.i. and in the majority of infected cells was severely decreased in amount by 48 hours p.i.. In a similar fashion, the levels of CSTL1, MFN1, KPNA2 and UBE2S in DENV-2 infected were examined by IFA. However for CSTL1, MFN1 and UBE2S, the specific antibodies available could either not detect the proteins of interest or reacted non-specifically with other proteins. For KPNA2, there appeared to be no difference in the amount or localization during DENV-2 infection compared to mock infected cells at 24 and 48 hours p.i.. This may have been due to an interaction of the anti-KPNA2 antisera with the smaller product detected in the Western blot analysis of KPNA2, masking any overall decrease in KPNA2 amounts. The analysis supported the Western blot analysis in that proteins that were observed to be significantly decreased during infection by Western blot analysis were also observed to decrease in virus infected cells when examined by IFA. Proteins that showed a smaller decrease were found to be more difficult to visualize by IFA although this was also dependent on the availability of highly specific antibodies. The biological validation identified two proteins that were severely decreased in DENV-2 infected cells, ERC1 and PRAF2. The decrease in the amounts of these proteins may be a direct or indirect effect of virus replication. ERC1 has previously been identified to interact with the DENV NS5 protein in a yeast two hybrid screen which we have also observed using a highthroughput co-immunoprecipitation analysis. siRNA knockdown of ERC1 inhibited the replication of a DENV replicon suggesting that ERC1 is required for efficient DENV replication which appears to contradict this study showing that ERC1 is decreased during DENV infection. However it may be that ERC1 plays different roles at various stages of the DENV lifecycle which illustrates how the use of different high-throughput approaches can complement one another to increase our understanding of the role of cellular proteins in the DENV lifecycle. Medicinal use of water in chronic kidney disease has gained research interest lately, as established efforts to retard CKD progression remain far from 20(S)-Notoginsenoside-R2 satisfactory. Epidemiological data associating fluid intake or urine volume with GFR decline in humans have not been fully conclusive. Nonetheless, there is increasing evidence linking fluid intake, vasopressin suppression and osmotic control with CKD and ADPKD progression. Kidney excretion is adjusted according to water and dietary solute intake, as well as water and solute losses by lungs, skin, and the gastrointestinal tract. The required urine volume can be determined by dividing the daily osmolar excretion, to maintain the body��s solute content at 9-methoxycamptothecine steady state, by the maximal urine osmolality, with failing kidneys losing capacity to concentrate urine maximally.
observably diminished in DENV-2 infected cells and by 48 hours p.i. was barely detectable. PRAF2 accumulated most strongly in the cytoplasm of the mock infected cells, showing a punctate distribution, supporting the previous observation that the protein was localized to the ER and transGolgi network. By contrast, in DENV-2 infected cells, PRAF2 appeared to co-localize with the viral E protein at 24 hours p.i. and in the majority of infected cells was severely decreased in amount by 48 hours p.i.. In a similar fashion, the levels of CSTL1, MFN1, KPNA2 and UBE2S in DENV-2 infected were examined by IFA. However for CSTL1, MFN1 and UBE2S, the specific antibodies available could either not detect the proteins of interest or reacted non-specifically with other proteins. For KPNA2, there appeared to be no difference in the amount or localization during DENV-2 infection compared to mock infected cells at 24 and 48 hours p.i.. This may have been due to an interaction of the anti-KPNA2 antisera with the smaller product detected in the Western blot analysis of KPNA2, masking any overall decrease in KPNA2 amounts. The analysis supported the Western blot analysis in that proteins that were observed to be significantly decreased during infection by Western blot analysis were also observed to decrease in virus infected cells when examined by IFA. Proteins that showed a smaller decrease were found to be more difficult to visualize by IFA although this was also dependent on the availability of highly specific antibodies. The biological validation identified two proteins that were severely decreased in DENV-2 infected cells, ERC1 and PRAF2. The decrease in the amounts of these proteins may be a direct or indirect effect of virus replication. ERC1 has previously been identified to interact with the DENV NS5 protein in a yeast two hybrid screen which we have also observed using a highthroughput co-immunoprecipitation analysis. siRNA knockdown of ERC1 inhibited the replication of a DENV replicon suggesting that ERC1 is required for efficient DENV replication which appears to contradict this study showing that ERC1 is decreased during DENV infection. However it may be that ERC1 plays different roles at various stages of the DENV lifecycle which illustrates how the use of different high-throughput approaches can complement one another to increase our understanding of the role of cellular proteins in the DENV lifecycle. Medicinal use of water in chronic kidney disease has gained research interest lately, as established efforts to retard CKD progression remain far from 20(S)-Notoginsenoside-R2 satisfactory. Epidemiological data associating fluid intake or urine volume with GFR decline in humans have not been fully conclusive. Nonetheless, there is increasing evidence linking fluid intake, vasopressin suppression and osmotic control with CKD and ADPKD progression. Kidney excretion is adjusted according to water and dietary solute intake, as well as water and solute losses by lungs, skin, and the gastrointestinal tract. The required urine volume can be determined by dividing the daily osmolar excretion, to maintain the body��s solute content at 9-methoxycamptothecine steady state, by the maximal urine osmolality, with failing kidneys losing capacity to concentrate urine maximally.
Synthase expression in heart and aorta were unchanged compared to controls
Endothelial dysfunction might be due to uncoupled eNOS. Several studies have shown that relaxin effects are endothelium and NO dependent. Thus, impaired endothelium mediated vascular relaxation in the dTGR model could explain the missing relaxin effectiveness. Recently Parikh et al. showed beneficial effects of relaxin on Eleutheroside-E atrial fibrillation in spontaneously hypertensive rats. Relaxin treatment reversed the transcripts for fibrosis, increasing conduction velocity, reduced electrophysiological abnormalities, and reversed atrial hypertrophy. In their study a one-week therapy was ineffective in suppressing atrial 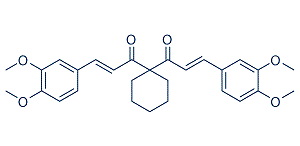 fibrillation and longer relaxin treatment was necessary. The authors speculate that reversal of fibrosis is a slow process as a result of the slow collagen turnover rate of around 5% per day in healthy hearts. The fibrosis in our dTGR is much more pronounced. This state-of-affairs and the fact that fibrosis accelerates over time might be the reason why relaxin was not successful in ameliorating target-organ damage in dTGR. We were also interested in the kidney in dTGR. Recently Yoshida et al demonstrated that relaxin protected against ischemia/reperfusion-induced renal injury by reducing apoptosis and inflammation. Relaxin also preserved renal function in their model. Their relaxin dose, namely 500 ng/h was higher than our high-dose. Moreover, the design of their study was based on acute renal failure; our study was chronic in nature. Parikh et al did not investigate inflammation, while Yoshida et al showed that the TNFa receptor-1 was up regulated in the kidney and normalized by relaxin. We found no such effects in dTGR model. The transcription factors nuclear factor-kB and activator-protein 1 are strongly activated and responsible for the sustained inflammatory and proliferative response in this model. Macrophages, dendritic cells and CD4 and CD8 T cells are strongly activated. Surface adhesion molecule expression, such as ICAM-1, VCAM-1, TNFa and interleukin-6, tissue factor production, and activation of enzymes producing reactive oxygen species are highly induced. We speculate that relaxins anti-inflammatory potential was not sufficient to counteract the pro-inflammatory storm in dTGR. In contrast to our findings, Lekgabe et al. showed that relaxin reduced target-organ damage in spontaneous hypertensive rats. However, end-organ damage takes 9�C10 months to develop in that model and is far less severe than the effects reported here. Relaxin, applied for 2 weeks, normalized fibrosis in heart and kidney, inhibited cell and increased MMP-2 expression. Blood pressure was not affected by relaxin treatment and mortality was not investigated. Wong et al investigated the effects of relaxin on fibrosis in streptozotocin -treated transgenic mRen-2 rats. That model is also Ang II-mediated and features fairly severe changes. Relaxin did not ameliorate glomerulopathy in this accelerated model of type 1 diabetes. Relaxin did not reduce Saikosaponin-B2 hypertension or albuminuria. Similar to our study, the authors were puzzled by the negative results. The authors observed that relaxin was very successful in reversing fibrosis when the TGF-b1 pathway was activated and subsequently phosphorylation and down regulation of smad-2 occurred. This was not the case in the dTGR.
fibrillation and longer relaxin treatment was necessary. The authors speculate that reversal of fibrosis is a slow process as a result of the slow collagen turnover rate of around 5% per day in healthy hearts. The fibrosis in our dTGR is much more pronounced. This state-of-affairs and the fact that fibrosis accelerates over time might be the reason why relaxin was not successful in ameliorating target-organ damage in dTGR. We were also interested in the kidney in dTGR. Recently Yoshida et al demonstrated that relaxin protected against ischemia/reperfusion-induced renal injury by reducing apoptosis and inflammation. Relaxin also preserved renal function in their model. Their relaxin dose, namely 500 ng/h was higher than our high-dose. Moreover, the design of their study was based on acute renal failure; our study was chronic in nature. Parikh et al did not investigate inflammation, while Yoshida et al showed that the TNFa receptor-1 was up regulated in the kidney and normalized by relaxin. We found no such effects in dTGR model. The transcription factors nuclear factor-kB and activator-protein 1 are strongly activated and responsible for the sustained inflammatory and proliferative response in this model. Macrophages, dendritic cells and CD4 and CD8 T cells are strongly activated. Surface adhesion molecule expression, such as ICAM-1, VCAM-1, TNFa and interleukin-6, tissue factor production, and activation of enzymes producing reactive oxygen species are highly induced. We speculate that relaxins anti-inflammatory potential was not sufficient to counteract the pro-inflammatory storm in dTGR. In contrast to our findings, Lekgabe et al. showed that relaxin reduced target-organ damage in spontaneous hypertensive rats. However, end-organ damage takes 9�C10 months to develop in that model and is far less severe than the effects reported here. Relaxin, applied for 2 weeks, normalized fibrosis in heart and kidney, inhibited cell and increased MMP-2 expression. Blood pressure was not affected by relaxin treatment and mortality was not investigated. Wong et al investigated the effects of relaxin on fibrosis in streptozotocin -treated transgenic mRen-2 rats. That model is also Ang II-mediated and features fairly severe changes. Relaxin did not ameliorate glomerulopathy in this accelerated model of type 1 diabetes. Relaxin did not reduce Saikosaponin-B2 hypertension or albuminuria. Similar to our study, the authors were puzzled by the negative results. The authors observed that relaxin was very successful in reversing fibrosis when the TGF-b1 pathway was activated and subsequently phosphorylation and down regulation of smad-2 occurred. This was not the case in the dTGR.
It is easy to perform in clinical practice and could be helpful in determining therapeutic regimens for GBM
Aminopeptidases exist widely in prokaryotic and eukaryotic microbial species, which can selectively catalyze the cleavage of the N-terminal amino acid residues from peptides and proteins. APs are associated with many human diseases and play an important role in a wide range of biological processes. The research to elucidate the catalytic mechanisms of APs is significant for medicine and pharmacology. APs have great application in various fields because of their broad substrate specificity, strict enantioselectivity, and high thermal stability. Besides being applied to synthesize pharmaceutical compounds and acting as versatile chiral building blocks, they are mainly applied in  the food industry. For instance, APs play an important role in debittering protein hydrolysate and increasing the content of free amino acids in protein hydrolysate. They are also used as additives in condiments, such as soy sauce and seasoning blends, to enhance the flavor and the nutritional value of foods. Based on the homology modeling and structure analysis of BSAP, we found that there was a protease-associated Gomisin-D domain in the non-catalytic region. The PA domain is about 150 amino acids long, containing two a-helices and seven b-sheets. Because this domain is found in several distinct Procyanidin-B1 classes of proteases, it was named PA domain. Besides the different protease families, this domain was also found in two classes of plant transmembrane proteins. However, the role of PA domain remains somewhat elusive. It has been speculated that PA domain was involved in protein-protein interaction. In M28 family, the PA domain was found in only a few APs and the role of PA domain in APs has not been reported yet. Herein we found that the PA domain showed much more flexibility than any other regions in BSAP by using molecular dynamics simulation. The structure of the hypothetical deleted form of BSAPDPA was modeled and analyzed using MDS, and it was predicted that deletion of this flexible domain can enhance the structure stability. In vivo, the enzymatic reaction is an important biological process that can serve a wide variety of purposes. Inappropriate activity of enzymes can be deleterious to the cell or the organism that produces them. Thus, many enzymes contain conserved domains that can serve as an auto-inhibitor, a substrate-targeting domain or a regulatory domain, allowing catalysis events to occur only in the correct subcellular compartment, at the correct time and with the correct substrates. However, in the industrial application of the enzymes, sometimes these regulatory domains were unnecessary or even detrimental to the catalytic process. In order to satisfy the industrial requirement, it was an efficient approach to reconstruct the enzymes via deletion of these redundant regions. It has been reported that truncation of the cellulose binding domain of glucanase improved its thermal stability. Recently, Xiangtao Liu et al reported that N-terminal truncation of a maleate cis-trans isomerase resulted in a highly active enzyme for the biocatalytic production of fumaric acid. The research about changing optimum pH of an invertase by N-terminal truncation was also reported.
the food industry. For instance, APs play an important role in debittering protein hydrolysate and increasing the content of free amino acids in protein hydrolysate. They are also used as additives in condiments, such as soy sauce and seasoning blends, to enhance the flavor and the nutritional value of foods. Based on the homology modeling and structure analysis of BSAP, we found that there was a protease-associated Gomisin-D domain in the non-catalytic region. The PA domain is about 150 amino acids long, containing two a-helices and seven b-sheets. Because this domain is found in several distinct Procyanidin-B1 classes of proteases, it was named PA domain. Besides the different protease families, this domain was also found in two classes of plant transmembrane proteins. However, the role of PA domain remains somewhat elusive. It has been speculated that PA domain was involved in protein-protein interaction. In M28 family, the PA domain was found in only a few APs and the role of PA domain in APs has not been reported yet. Herein we found that the PA domain showed much more flexibility than any other regions in BSAP by using molecular dynamics simulation. The structure of the hypothetical deleted form of BSAPDPA was modeled and analyzed using MDS, and it was predicted that deletion of this flexible domain can enhance the structure stability. In vivo, the enzymatic reaction is an important biological process that can serve a wide variety of purposes. Inappropriate activity of enzymes can be deleterious to the cell or the organism that produces them. Thus, many enzymes contain conserved domains that can serve as an auto-inhibitor, a substrate-targeting domain or a regulatory domain, allowing catalysis events to occur only in the correct subcellular compartment, at the correct time and with the correct substrates. However, in the industrial application of the enzymes, sometimes these regulatory domains were unnecessary or even detrimental to the catalytic process. In order to satisfy the industrial requirement, it was an efficient approach to reconstruct the enzymes via deletion of these redundant regions. It has been reported that truncation of the cellulose binding domain of glucanase improved its thermal stability. Recently, Xiangtao Liu et al reported that N-terminal truncation of a maleate cis-trans isomerase resulted in a highly active enzyme for the biocatalytic production of fumaric acid. The research about changing optimum pH of an invertase by N-terminal truncation was also reported.